- 1Department of Internal Medicine, Harbin Medical University Cancer Hospital, Harbin Medical University, Harbin, Heilongjiang, China
- 2Department of Radiology, Harbin Medical University Cancer Hospital, Harbin Medical University, Harbin, Heilongjiang, China
- 3Department of Science and Education, School of Computer Science and Technology, Harbin University of Science and Technology, Harbin, Heilongjiang, China
- 4Department of Gastrointestinal Medical Oncology, Harbin Medical University Cancer Hospital, Harbin Medical University, Harbin, Heilongjiang, China
Background: Systemic inflammation and glucose metabolism have been closely related to the survival of cancer patients. Therefore, we aimed to evaluate whether preoperative glucose-to-lymphocyte ratio (GLR) can be used to predict the survival of cancer patients.
Methods: We retrospectively examined 2172 cancer patients who underwent surgery from January 1, 2014, to December 31, 2016. There were 240 patients with non-small cell lung cancer (NSCLC), 378 patients with colorectal cancer (CRC), 221 patients with breast cancer (BC), 335 patients with gastric cancer (GC), 270 patients with liver cancer, 233 patients with esophageal cancer (EC), 295 patients with renal cancer, and 200 patients with melanoma. The formula for preoperative GLR calculation was as follows: GLR=glucose/lymphocyte count. The overall survival (OS) was estimated using the Kaplan-Meier method. The predictive factors for OS were determined using multivariate analysis.
Results: The Kaplan-Meier analysis showed that the median survival time in the high-GLR group was much shorter than that of those in the low-GLR group for different cancers. Cox multivariate regression analysis reveals that preoperative GLR was an independent factor for predicting overall survival in different tumor types.
Conclusion: Elevated preoperative GLR was remarkably associated with a poorer prognosis in patients with NSCLC, CRC, breast cancer, gastric cancer, kidney cancer, liver cancer, esophageal cancer, and melanoma. Preoperative GLR promises to be an essential predictor of survival for cancer patients.
Introduction
As the morbidity rate continues to climb, cancer is not only a major public health problem but also one of the leading contributors to death in the world (1). Up to date, surgery resection is still the mainstay of curative treatment options for most tumors (2). However, despite efforts to develop new surgical strategies, overall survival is still unsatisfactory. Therefore, a more accurate evaluation index to predict the long-term survival of patients has high clinical value.
Diabetes mellitus (DM) and cancer are two prevalent disorders that coexist, and the incidence and prevalence of both are rising (3). DM and hyperglycemia have been demonstrated to have significant impacts on the incidence and prognosis of cancer (4–6). Moreover, the metabolic abnormalities in hyperglycemia and diabetes substantially contribute to the development and progression of cancer (7). A meta-analysis revealed that metformin is an independent protective factor for cancer risk in DM patients (8). In addition, large bodies of accumulated research have also confirmed that the development and progression of cancer increase the risk of diabetes (9).
At the same time, lymphocytes, being one of the crucial components of the systemic inflammatory response, are engaged in cell-mediated antitumor responses (10). Furthermore, its profound role in immune surveillance that protects the host from tumor development has also been observed in mice and humans (11).
The available literature demonstrated the potential association of glucose-to-lymphocyte ratio (GLR) with prognosis in gallbladder, colorectal cancer (CRC), and pancreatic cancer (12). However, there are relatively few studies on the prognostic association of GLR with other tumors. The objective of our study is to evaluate the prognostic role of preoperative GLR in patients with gastric cancer (GC), renal carcinoma, colorectal cancer, non-small cell lung cancer, breast cancer (BC), liver cancer, esophageal cancer (EC), and melanoma.
Patients and methods
Study population
We reviewed the clinical information of 2172 cancer patients who underwent curative resection at the Harbin Medical University Cancer Hospital between January 1, 2014, and December 31, 2016. There were 240 patients with non-small cell lung cancer, 378 patients with colorectal cancer, 221 patients with breast cancer, 335 patients with gastric cancer, 270 patients with liver cancer, 233 patients with esophageal cancer, 295 patients with renal cell cancer, and 200 patients with melanoma. Patients were included according to the following criteria: (1) pathologically confirmed evidence of each cancer, (2) completed preoperative blood tests involving fasting glucose and lymphocyte counts, and (3) followed for more than 60 months. Exclusion criteria for patients were as follows: (1) they had received antitumor therapy before surgery; (2) they had a history of other primary malignancies; (3) they had acute inflammatory disease; and (4) they failed to follow up.
Overall survival (OS) was defined from the date of surgery to the date of death or the date of the last follow-up. All patients were followed up by telephone once every 3-6 months. The cut-off date for follow-up evaluations is December 31, 2021. The survival data was derived from medical records and telephone follow-ups. And the work has been reported in line with the REMARK criteria (13). Patients’ demographic characteristics and laboratory parameters were extracted from their electronic medical records. All laboratory parameters were assayed within a week before the operation. The formula for preoperative GLR calculation was as follows: GLR=fasting blood glucose (mmol/L)/lymphocyte count (×109/L).
This research was in strict compliance with the Helsinki Declaration. This study was approved by our Institutional Review Board (approval number KY2022-10). Since it was a retrospective study, we waived informed consent.
Statistical analysis
Statistical tests were performed with SPSS version 25.0 software (SPSS Inc., Chicago, IL, USA). Receiver operating characteristic (ROC) curves were constructed using MedCalc version 15.0 software to assign cut-off values for GLR levels as well as sensitivities and specificities. The Kolmogorov-Smirnov test was used to determine if the data were normally distributed. T-tests were utilized for the comparison of normally distributed continuous variables, while categorical variables were compared with chi-square tests. The Kaplan-Meier method was used to derive OS, and the results were compared with the log-rank test. Multivariate analysis was conducted using the Cox proportional hazards regression model to estimate the independent predictors of OS. The proportional-hazards assumption was examined before Cox regression analysis. Univariate and multivariate Cox regression analyses were carried out to determine the hazard ratio (HR) and the corresponding 95% confidence interval (CI). Variables with a p value of < 0.10 in the univariate analysis were subjected to multivariate analysis. All reported p values were two-sided, and p values < 0.05 were regarded as statistically significant.
Results
Among the 2172 patients collected, the mean age was 55.72 years (range 10-87), 1265 (58.2%) were men, and 907 (41.8%) were women.
The patient’s clinical characteristics based on preoperative GLR levels are summarized in Table 1. In gastric cancer, colorectal cancer, liver cancer, esophageal cancer, and renal cancer, lower hemoglobin and platelet count were likely to appear in the high-GLR group. In non-small cell lung cancer, colorectal cancer, and renal cancer, age in the two groups showed a significant difference. Moreover, high GLR levels were correlated with white blood cell in melanoma, breast cancer, liver cancer, esophageal cancer, renal cancer, and non-small cell lung cancer.
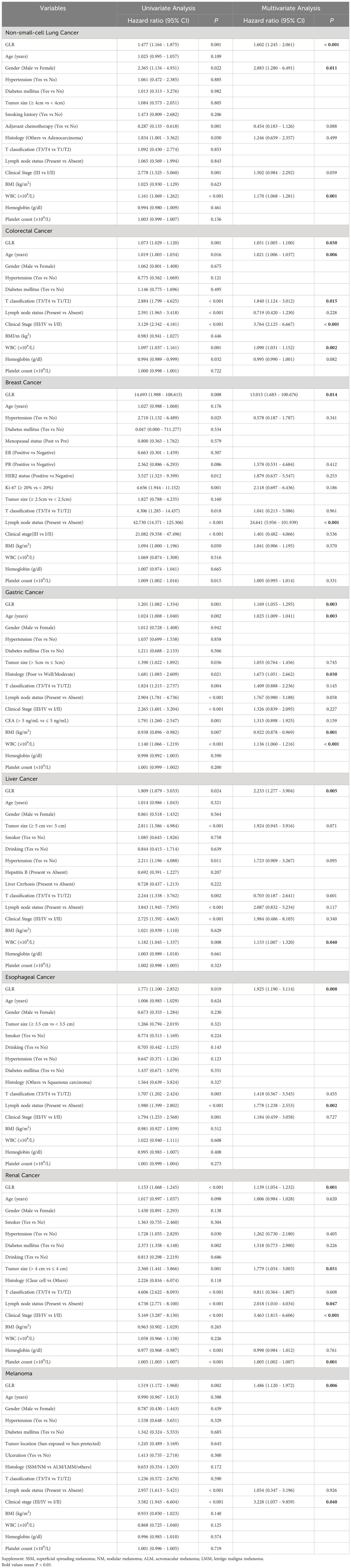
A ROC curve analysis was constructed to determine the optimal cutoff value for GLR in different tumor types (Figure 1). Based on the analysis of receiver operating characteristic curves, the optimal GLR cut-off values for gastric, renal, colorectal, non-small cell lung, breast, liver, esophageal, and melanoma cancers were 4.1, 2.53, 6.17, 3.27, 3.2, 4.08, 3.46, and 3.5, respectively. And the corresponding sensitivity and specificity are shown in Figure 1. Patients were classified as having high or low preoperative GLR according to cut-off values. We found that elevated GLR significantly predicted overall survival (Figure 2). Among patients with non-small cell lung cancer, 72 (30%) had higher preoperative GLR levels. With a median follow-up of 60 months, 43 (17.9%) patients had death events. 22 patients with GLR > 3.27 and 21 patients with GLR ≤ 3.27 had death events. Overall survival was significantly shorter in patients with high GLR (n=72) versus those with low GLR (n=168) (p < 0.001). The mean survival time was 45.5 months for patients with GLR > 3.27 and 53.4 months for patients with GLR ≤ 3.27, respectively. Kaplan-Meier OS curves for normal versus increased GLR showed a notable separation (Figure 2A). In patients with colorectal cancer, there were 212 (56.1%) patients who had death events. Compared to those with low GLR levels, the patients with high GLR levels had significantly shorter overall survival (survival rates of 21.2% and 46.1%, respectively, p < 0.001; Figure 2B). In breast cancer, OS was lower in high-GLR subjects than in low-GLR counterparts (mean survival time, 54.1 months vs 55.9 months, p < 0.001; Figure 2C). In gastric cancer, the OS rate was markedly worse in the high-GLR group than that in the low-GLR group (5-year survival rates of 32.3% and 53.1%, respectively, p < 0.001; Figure 2D). In liver cancer, OS was lower in high-GLR subjects than that in low-GLR counterparts (mean survival time, 27.3 months vs 30.6 months, p = 0.027; Figure 2E). Among patients with renal cancer, the high GLR grade group had a worse OS than the low GLR grade group (mean survival time, 46.1 months vs 54.3 months, p < 0.001; Figure 2G). Similarly, in melanoma, subjects with a high GLR have a shorter OS compared to patients with a lower GLR (mean survival time, 44.9 months vs 52.8 months, p = 0.005; Figure 2H). And in esophageal cancer, OS was lower in high-GLR subjects than in low-GLR subjects (mean survival time, 34.7 months vs 43.9 months, p = 0.017; Figure 2F).
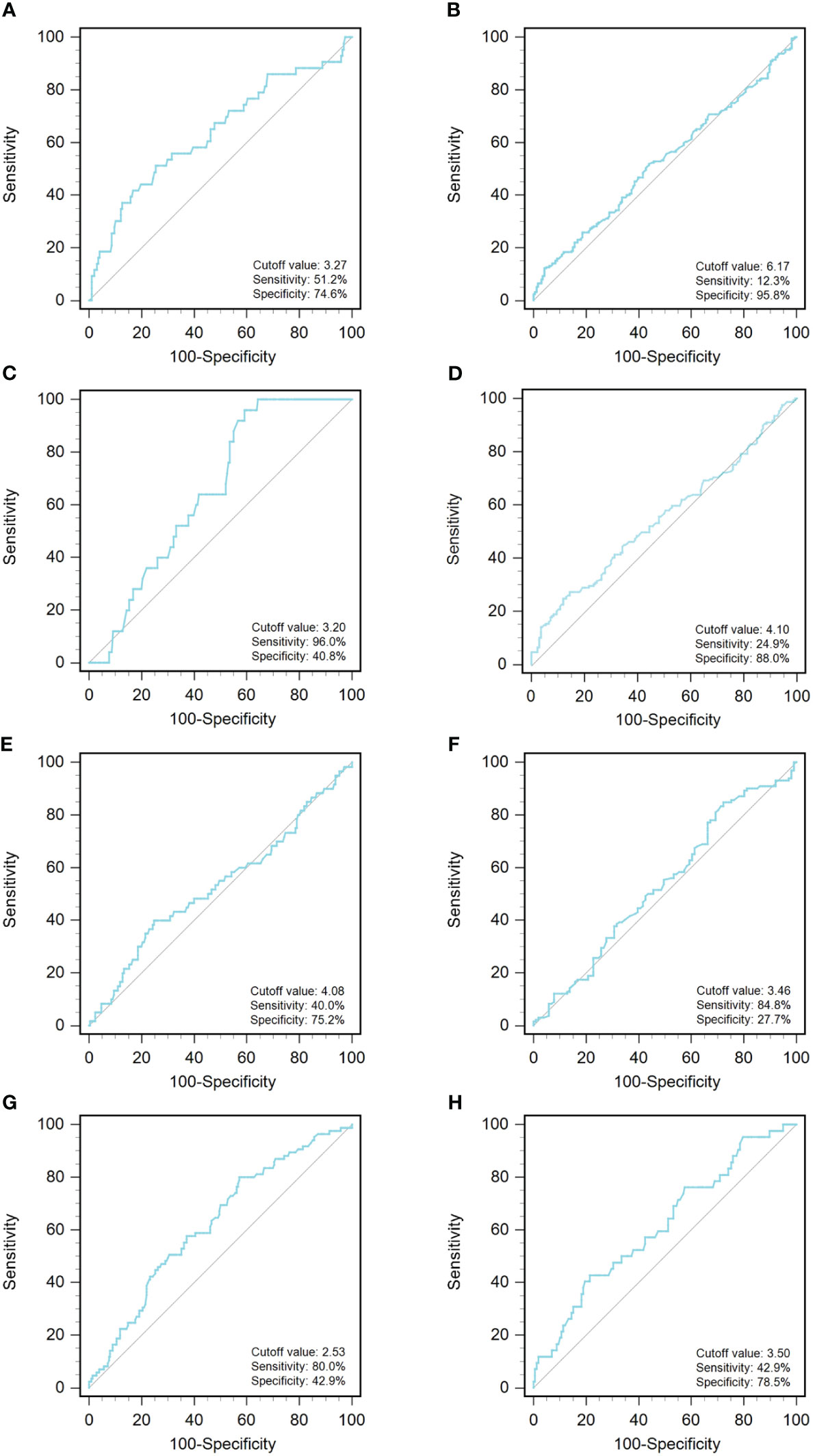
Figure 1 An optimized cut-off value was determined for preoperative GLR using ROC curve analysis. The ROC curve identified the optimal cutoff value of GLR with sensitivity and specificity. (A) non-small-cell lung cancer; (B) colorectal cancer; (C) breast cancer; (D) gastric cancer; (E) liver cancer; (F) esophageal cancer; (G) renal cancer; and (H) melanoma. ROC curve, receiver operating characteristic curve; GLR, glucose to lymphocyte ratio.
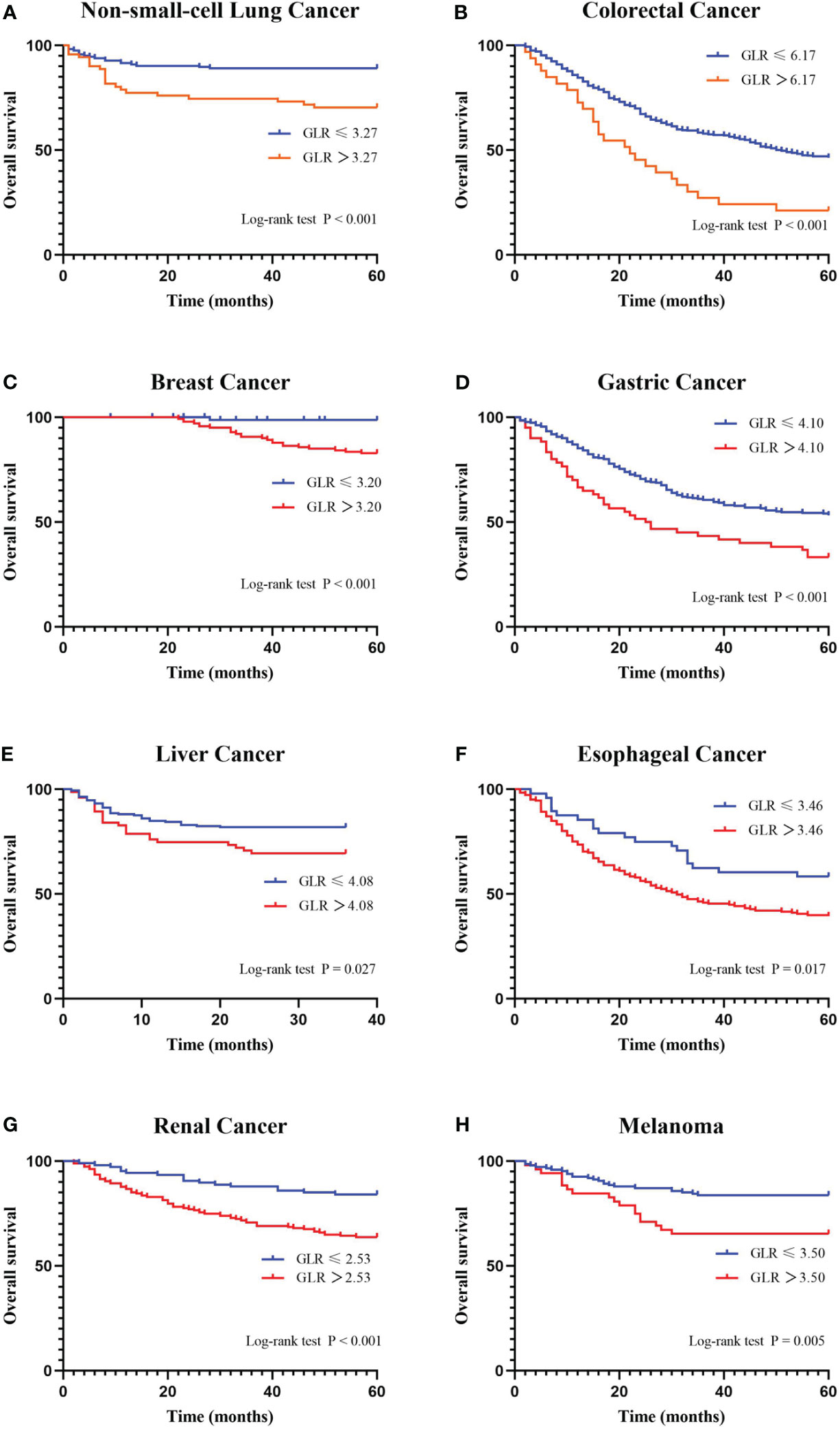
Figure 2 Kaplan-Meier curves for overall survival stratifed by preoperative GLR. Overall survival Kaplan-Meier survival curves according to GLR levels for patients who underwent radical surgery. The 5-year overall survival in patients with high GLR or low GLR is plotted. Kaplan-Meier analysis demonstrated that high GLR was significantly associated with the shorter overall survival. (A) non-small-cell lung cancer; (B) colorectal cancer; (C) breast cancer; (D) gastric cancer; (E) liver cancer; (F) esophageal cancer; (G) renal cancer; and (H) melanoma. GLR, glucose to lymphocyte ratio.
The univariate and multivariate analyses were performed to evaluate the preoperative predictors for OS (Table 2). According to the univariate analysis, GLR, gender, adjuvant chemotherapy, histology, clinical stage, and white blood cell were significantly correlated with OS in patients with NSCLC. In colorectal cancer, GLR, age, T classification, lymph node status, clinical stage, hemoglobin, and white blood cell were related to OS. In gastric cancer, GLR, age, tumor size, histology, T classification, lymph node status, clinical stage, carcinoma embryonic antigen (CEA), BMI, and white blood cell were in correlation with OS. In patients with renal cancer, GLR, age, hypertension, diabetes mellitus, tumor size, T classification, lymph node status, clinical stage, hemoglobin, and platelet count were significantly related to OS. In melanoma, GLR, lymph node status, and clinical stage were prognostic‐related risk factors for OS. In patients with liver cancer, GLR, hypertension, tumor size, T classification, lymph node status, clinical stage, and white blood cell were related to OS. In esophageal cancer, GLR, T classification, lymph node status, and clinical stage were significantly related to OS. And, in breast cancer, GLR, progesterone receptor (PR), human epidermal growth factor receptor-2 (HER-2), Ki-67, T classification, lymph node status, clinical stage, hypertension, BMI, and platelet count were significantly related to OS. Next, the variables showing statistical significance in the univariate analysis (p < 0.10) were included in the multivariate analysis. In multivariate analysis, GLR was identified as an independent prognostic factor for OS in different tumor types.
Discussion
In this study, we retrospectively analyzed the predictive value of preoperative GLR in patients with CRC, NSCLC, GC, EC, BC, renal cancer, liver cancer, and melanoma. It was found that increased GLR was markedly associated with shorter OS.
Previous studies have proven that GLR is a prognostic marker for some tumors, such as CRC (14), pancreatic carcinoma (12) and PT2 gallbladder carcinoma (15). Our study was consistent with the above results. In addition, our results showed the prognostic value of preoperative GLR in other cancers. Consistent with previous studies (16–19), our findings confirmed that age, BMI, WBC, and platelet count were independently associated with OS in the multivariate analysis in some cancers.
GLR is derived from the ratio of blood glucose to lymphocyte count (20). Altered glucose metabolism is a marked trait of cancer. Therefore, it is worth considering that tumor cell glycolytic activity increases when blood glucose is elevated, and then cancer cells transport extracellular glucose through the cytoplasm, leading to an increase in intracellular glucose, whose fermentation into lactic acid generates energy that activates cellular signaling pathways, thereby mediating the spread, invasion, and metastasis of cancer cells (21). It has been confirmed that the diabetes caused by hyperglycemia gives rise to hyperinsulinemia and insulin resistance, which may lead to changes in the tumor microenvironment by producing irreversible glycation end products or by affecting the expression of angiogenic factors and the acidity of the microenvironment, promoting tumor development, and even increasing tumor metastasis and resistance to chemotherapy (22–24). Also, the abysmal outcome of hyperglycemia is associated with chronic subclinical inflammation, referred to as “meta-inflammation”. Chronic subclinical inflammation exacerbates hyperglycemia by modulating insulin resistance, leading to a series of diabetic complications, while hyperglycemia promotes the production of free radicals, leading to inflammation and metabolic disorders, thus creating a vicious cycle that exacerbates disease progression (25, 26). These form the basis of a poorer prognosis for tumor patients. Moreover, lymphocytes have an essential role in immune regulation and the prevention of tumor development. On the one hand, lymphocytes suppress cancer progression by inhibiting cell proliferation and promoting cell death (24). Several reports have revealed that lymphocytes can activate a cell-mediated immune response and stimulate the release of cytokines such as interferon and TNF-α to exert organismal protective effects, even leading to the lysis of tumor cells (27–29). On the other hand, cumulative evidence demonstrated that lymphocytes could indicate the nutritional status of patients (30). In brief, elevated GLR, that is, high glucose and low lymphocyte count, is strongly associated with cancer progression and worse OS, which is in accordance with our findings.
Compared with the existing studies, this research involved a wide range of diseases, and the results were more comprehensive. However, our research had some limitations. Firstly, the study has a retrospective design and the sample size was not large enough. Secondly, the potential confounders that may exist (e.g., drug administration, patient selection, and surgical procedures) may have caused the sampling error. Thirdly, the cut-off values for specific cancer types are required for further evaluation in the future. Finally, further investigation is needed regarding the mechanisms at the molecular level. Moreover, serum lactate and inflammatory cytokines, such as TNFα or IL-10, should be detected in future studies.
GLR is a simple, cost-effective, and noninvasive parameter in clinical practice. Our study revealed the prognostic value of preoperative GLR in some resectable tumors. Future prospective studies are required to confirm the findings. Moreover, it would be interesting to investigate whether adding GLR to other prognosis scores could improve their performance.
In conclusion, elevated preoperative GLR was remarkably associated with a poorer prognosis in patients with NSCLC, CRC, breast cancer, gastric cancer, kidney cancer, liver cancer, esophageal cancer, and melanoma. Preoperative GLR promises to be an essential predictor of survival for cancer patients.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
This research was in strict compliance with the Helsinki Declaration. This study was approved by our Institutional Review Board (approval number KY2022-10). As the study was retrospective, written informed consent was waived.
Author contributions
LL: Data curation, Writing – original draft. BZ: Investigation, Writing – original draft, Data analysis. RW: Formal analysis, Methodology, Writing – review & editing. WH: Data curation, Investigation, Software, Writing – review & editing. YN: Conceptualization, Supervision, Validation, Writing – review & editing. WW: Investigation, Validation, Visualization, Writing – review & editing. QJ: Data curation, Methodology, Supervision, Writing – original draft. JY: Formal analysis, Validation, Visualization, Writing – review & editing. GW: Conceptualization, Methodology, Writing – review & editing. SM: Software, Supervision, Validation, Writing – review & editing. YL: Formal analysis, Investigation, Visualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article. This study did not receive any specific funding from public, commercial or non-profit sector funding agencies. None of the authors have any financial and relevant financial and personal relationships with other people or organisations to disclosure in this paper.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. (2022) 7:33. doi: 10.3322/caac.21708.
2. Wyld L, Audisio RA, Poston GJ. The evolution of cancer surgery and future perspectives. Nat Rev Clin Oncol. (2015) 115:124. doi: 10.1038/nrclinonc.2014.191.
3. Magliano DJ, Sacre JW, Harding JL, Gregg EW, Zimmet PZ, Shaw JE. Young-onset type 2 diabetes mellitus - implications for morbidity and mortality. Nat Rev Endocrinol. (2020) 321:331. doi: 10.1038/s41574-020-0334-z.
4. Barone BB, Yeh HC, Snyder CF, Peairs KS, Stein KB, Derr RL, et al. Long-term all-cause mortality in cancer patients with preexisting diabetes mellitus: a systematic review and meta-analysis. JAMA. (2008) 2754:2764. doi: 10.1001/jama.2008.824.
5. Suh S, Kim KW. Diabetes and cancer: cancer should be screened in routine diabetes assessment. Diabetes Metab J. (2019) 733:743. doi: 10.4093/dmj.2019.0177.
6. Tsilidis KK, Kasimis JC, Lopez DS, Ntzani EE, Ioannidis JP. Type 2 diabetes and cancer: umbrella review of meta-analyses of observational studies. BMJ. (2015) 350:g7607. doi: 10.1136/bmj.g7607.
7. Bánhegyi RJ, Gazdag A, Rácz B, Beke S, Fülöp N, Onkodiabetológia I. Metabolikus és molekuláris összefüggések a rosszindulatú daganatok és a cukorbetegség között [Oncodiabetology I. Metabolic and molecular relationships between cancer and diabetes]. Orv Hetil. (2022) 163(39):1535–43. doi: 10.1556/650.2022.32564
8. Zhang K, Bai P, Dai H, Deng Z. Metformin and risk of cancer among patients with type 2 diabetes mellitus: A systematic review and meta-analysis. Prim Care Diabetes. (2021) 52:58. doi: 10.1016/j.pcd.2020.06.001.
9. Hwangbo Y, Kang D, Kang M, Kim S, Lee EK, Kim YA, et al. Incidence of diabetes after cancer development: A korean national cohort study. JAMA Oncol. (2018) 1099:1105. doi: 10.1001/jamaoncol.2018.1684.
10. Paijens ST, Vledder A, De Bruyn M, Nijman HW. Tumor-infiltrating lymphocytes in the immunotherapy era. Cell Mol Immunol. (2021) 842:859. doi: 10.1038/s41423-020-00565-9.
11. Wu SY, Fu T, Jiang YZ, Shao ZW. Natural killer cells in cancer biology and therapy. Mol Cancer. (2020) 19(1):120. doi: 10.1186/s12943-020-01238-x.
12. Zhong A, Cheng CS, Kai J, Lu R, Guo L. Clinical significance of glucose to lymphocyte ratio (GLR) as a prognostic marker for patients with pancreatic cancer. Front Oncol. (2020) 520330. doi: 10.3389/fonc.2020.520330.
13. McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM, et al. Reporting recommendations for tumor marker prognostic studies (REMARK). J Natl Cancer Inst. (2005) 1180:1184. doi: 10.1093/jnci/dji237.
14. Yang M, Zhang Q, Ge Y, Tang M, Zhang X, Song M, et al. Glucose to lymphocyte ratio predicts prognoses in patients with colorectal cancer [published online ahead of print, 2022 Dec 7]. Asia Pac J Clin Oncol. (2022) 19(4):542–8. doi: 10.1111/ajco.13904.
15. Navarro J, Kang I, Hwang HK, Yoon DS, Lee WJ, Kang CW. Glucose to lymphocyte ratio as a prognostic marker in patients with resected pT2 gallbladder cancer. J Surg Res. (2019) 17:29. doi: 10.1016/j.jss.2019.02.043.
16. Laconi E, Marongiu F, Degregori J. Cancer as a disease of old age: changing mutational and microenvironmental landscapes. Br J Cancer. (2020) 943:52. doi: 10.1038/s41416-019-0721-1.
17. Schwartz SS, Grant S FA, Herman ME. Intersections and clinical translations of diabetes mellitus with cancer promotion, progression and prognosis. Postgrad Med. (2019) 597:606. doi: 10.1080/00325481.2019.1657358.
18. Smeda M, Przyborowski K, Stojak M, Chlopicki S. The endothelial barrier and cancer metastasis: Does the protective facet of platelet function matter? Biochem Pharmacol. (2020) 176:113886. doi: 10.1016/j.bcp.2020.113886.
19. Xu H, Zheng X, Ai J, Yang L. Hemoglobin, albumin, lymphocyte, and platelet (HALP) score and cancer prognosis: A systematic review and meta-analysis of 13,110 patients. Int Immunopharmacol. (2023) 114:109496. doi: 10.1016/j.intimp.2022.109496.
20. Li L, Zou G, Liu J. Preoperative glucose-to-lymphocyte ratio is an independent predictor for acute kidney injury after cardiac surgery in patients in intensive care unit. Int J Gen Med. (2021) 6529:6537. doi: 10.2147/IJGM.S335896.
21. Wahdan-Alaswad R, Fan Z, Edgerton SM, Liu B, Deng XS, Arnadottir SS, et al. Glucose promotes breast cancer aggression and reduces metformin efficacy. Cell Cycle. (2013) 3759:3769. doi: 10.4161/cc.26641.
22. Chott A, Sun Z, Morganstern D, Pan J, Li T, Susani M, et al. Tyrosine kinases expressed in vivo by human prostate cancer bone marrow metastases and loss of the type 1 insulin-like growth factor receptor. Am J Pathol. (1999) 1271:1279. doi: 10.1016/S0002-9440(10)65229-7.
23. Li W, Zhang X, Sang H, Zhou Y, Shang C, Wang Y, et al. Effects of hyperglycemia on the progression of tumor diseases. J Exp Clin Cancer Res. (2019) 38(1):327. doi: 10.1186/s13046-019-1309-6.
24. Siska PJ, Rathmell JC. T cell metabolic fitness in antitumor immunity. Trends Immunol. (2015) 257:264. doi: 10.1016/j.it.2015.02.007.
25. Berbudi A, Rahmadika N, Tjahjadi AI, Ruslami R. Type 2 diabetes and its impact on the immune system. Curr Diabetes Rev. (2020) 442:449. doi: 10.2174/1573399815666191024085838.
26. De Heredia FP, Gómez-Martínez S, Marcos A. Obesity, inflammation and the immune system. Proc Nutr Soc. (2012) 332:338. doi: 10.1017/S0029665112000092.
27. Greten FP, Grivennikov SI. Inflammation and cancer: triggers, mechanisms, and consequences. Immunity. (2019) 27:41. doi: 10.1016/j.immuni.2019.06.025.
28. Koliaraki V, Prados A, Armaka M, Kollias G. The mesenchymal context in inflammation, immunity and cancer. Nat Immunol. (2020) 974:982. doi: 10.1038/s41590-020-0741-2.
29. Lin DZ, Qu N, Shi RL, Lu ZW, Ji QH, Wu WL. Risk prediction and clinical model building for lymph node metastasis in papillary thyroid microcarcinoma. Onco Targets Ther. (2016) 5307:5316. doi: 10.2147/OTT.
30. Reljic D, Herrmann HJ, Neurath MF, Zopf Y. Iron Beats Electricity: Resistance Training but Not Whole-Body Electromyostimulation Improves Cardiometabolic Health in Obese Metabolic Syndrome Patients during Caloric Restriction-A Randomized-Controlled Study. Nutrients. (2021) 13(5):1604. doi: 10.3390/nu13051640.
Keywords: cancer, survival, prognosis, glucose to lymphocyte ratio, lung cancer
Citation: Liu L, Zhang B-b, Li Y-z, Huang W-j, Niu Y, Jia Q-c, Wang W, Yuan J-r, Miao S-d, Wang R-t and Wang G-y (2024) Preoperative glucose-to-lymphocyte ratio predicts survival in cancer. Front. Endocrinol. 15:1284152. doi: 10.3389/fendo.2024.1284152
Received: 28 August 2023; Accepted: 06 February 2024;
Published: 04 March 2024.
Edited by:
Princy Francis, Mayo Clinic, United StatesReviewed by:
Jesus Rico-Feijoo, Hospital Universitario Río Hortega, SpainMeng Zhou, Wenzhou Medical University, China
Chuanfu Li, East Tennessee State University, United States
Guochang Hu, University of Illinois Chicago, United States
Copyright © 2024 Liu, Zhang, Li, Huang, Niu, Jia, Wang, Yuan, Miao, Wang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rui-tao Wang, ruitaowang@126.com; Guang-yu Wang, guangyuwang@hrbmu.edu.cn
†These authors have contributed equally to this work and share first authorship
 Le Liu
Le Liu Bei-bei Zhang1†
Bei-bei Zhang1† Wen-juan Huang
Wen-juan Huang Qing-chun Jia
Qing-chun Jia Jia-rui Yuan
Jia-rui Yuan Shi-di Miao
Shi-di Miao Rui-tao Wang
Rui-tao Wang