- 1Division of Pancreatic Surgery, Department of General Surgery, Qilu Hospital of Shandong University, Jinan, Shandong, China
- 2Department of Pathology, Qilu Hospital of Shandong University, Jinan, Shandong, China
Background: CXC chemokine receptor 4 (CXCR4) is associated with the progression and metastasis of numerous malignant tumors. However, its relationship with Gastroenteropancreatic Neuroendocrine Neoplasms Grade 3 (GEP-NENs G3) is unclear. The aim of this study was to characterize the expression of CXCR4 in GEP-NENS and to explore the clinical and prognostic value of CXCR4.
Methods: This study retrospectively collected clinical and pathological data from patients with GEP-NENs who receiving surgery in Qilu Hospital of Shandong University from January 2013 to April 2021, and obtained the overall survival of the patients based on follow-up. Immunohistochemistry (IHC) was performed on pathological paraffin sections to observe CXCR4 staining. Groups were made according to pathological findings. Kaplan-Meier (K-M) curve was used to evaluate prognosis. SPSS 26.0 was used for statistical analysis.
Results: 100 GEP-NENs G3 patients were enrolled in this study. There was a significant difference in primary sites (P=0.002), Ki-67 index (P<0.001), and Carcinoembryonic Antigen (CEA) elevation (P=0.008) between neuroendocrine tumor (NET) G3 and neuroendocrine carcinoma (NEC). CXCR4 was highly expressed only in tumors, low or no expressed in adjacent tissues (P<0.001). The expression level of CXCR4 in NEC was significantly higher than that in NET G3 (P=0.038). The K-M curves showed that there was no significant difference in overall survival between patients with high CXCR4 expression and patients with low CXCR4 expression, either in GEP-NEN G3 or NEC (P=0.920, P=0.842. respectively).
Conclusion: Differential expression of CXCR4 was found between tumor and adjacent tissues and between NET G3 and NEC. Our results demonstrated that CXCR4 can be served as a new IHC diagnostic indicator in the diagnosis and differential diagnosis of GEP-NENs G3. Further studies with multi-center, large sample size and longer follow-up are needed to confirm the correlation between CXCR4 expression level and prognosis.
1 Introduction
Neuroendocrine neoplasms (NENs) are highly heterogeneous solid tumors originating from the diffuse neuroendocrine system. They have neuroendocrine markers and can produce hormones such as peptides and biogenic amines (1, 2). Gastroenteropancreatic neuroendocrine neoplasms (GEP-NENs) accounts for about 60% of all NENs (3). The incidence of NENs has increased remarkably in recent years. Statistics from the Surveillance, Epidemiology, and End Results Database (SEER) showed that the incidence of GEP-NENs increased from 1.09/100,000 in 1973 to 6.98/100,000 in 2012 (4).
The classification of NENs is based on tumor proliferation activity (including Ki-67 index and mitotic rate), pathological morphology, and immunohistochemical (IHC) staining. 2019 WHO Classification of Digestive System Tumors (5th Edition) updated and defined a new subtype, GEP-NET G3. NENs can be classified into G1, G2 and G3 based on tumor proliferation activity, and G3 includes well-differentiated NET G3 and poorly-differentiated NEC (5). Few studies have been carried out focusing on the similarities and differences between well-differentiated NET G3 and poorly-differentiated NEC up to now. There are significant differences between NET G3 and NEC in terms of treatment. For instance, the European Society for Medical Oncology considers locally advanced NEC or NEC with distant metastasis as a contraindication for surgery, but NET G3 can be considered for surgical treatment (6). Therefore, accurate identification of GEP-NET G3 and NEC is of great significance for the selection of following treatment. However, the accuracy of existing immunohistochemical diagnostic indicators such as Rb, P53, DAXX/ATRX, and SSTR2 in the identification of NET G3 and NEC is not satisfactory (7–9).
Chemokines are a kind of small protein peptides that have directional chemotactic effects on neutrophils and monocytes. CXC chemokine receptor 4 (CXCR4), which belongs to the CXCR subfamily and is a G protein-coupled sevenfold transmembrane receptor. The coding gene is located on chromosome 2q21. Its N-terminal is located extracellular and is the binding site of the only ligand CXCL12 (CXC chemokine Ligand 12), while its C-terminal is located intracellular and contains Ser/Thr site, which is involved in intracellular signal transduction (10, 11). CXCR4 is associated with the occurrence, progression, invasion, and metastasis of a variety of malignant tumors according to previous literatures (12–16). CXCR4 has also been considered a prognostic factor in NENs, its expression level correlates with the malignancy of NENs (17, 18). In a retrospective study, Popa et al. found that high CXCR4 expression was associated with high-grade advanced NENs and tumor metastasis (18).
This study aimed to detect the expression pattern of CXCR4 in patients with NET G3 and NEC, to correlate the level of expression with clinicopathological characteristics and survival, and to provide preliminary validation results for the diagnosis, differential diagnosis, prognosis prediction and targeted therapy of GEP-NEN G3.
2 Methods
2.1 Study design
This study was a single-center, retrospective, observational study involving patients with GEP-NENs G3 who were admitted to Qilu Hospital of Shandong University from January 2013 to April 2021. We comprehensively collected patient information, including (1) Clinical data: age, gender, tumor markers. (2) Pathological data: tumor primary site, tumor size, stage. (3) Prognostic data: Overall survival (OS) time was defined as the time from surgery to death from any cause. At the same time, IHC staining of tumor marker CXCR4 was performed on the pathological specimens of all patients for further statistical analysis. The 2019 WHO Classification of Digestive System (5th Edition) was used for diagnosis and staging of NENs (5). By retrospectively collecting pathological data, GEP-NENs were classified into NET G3 and NEC by two experienced pathologists. This research has passed the review of the Ethics Committee of Qilu Hospital of Shandong University. The Study was Conducted in accordance with the Declaration of Helsinki (revised in 2013).
2.2 Case selection
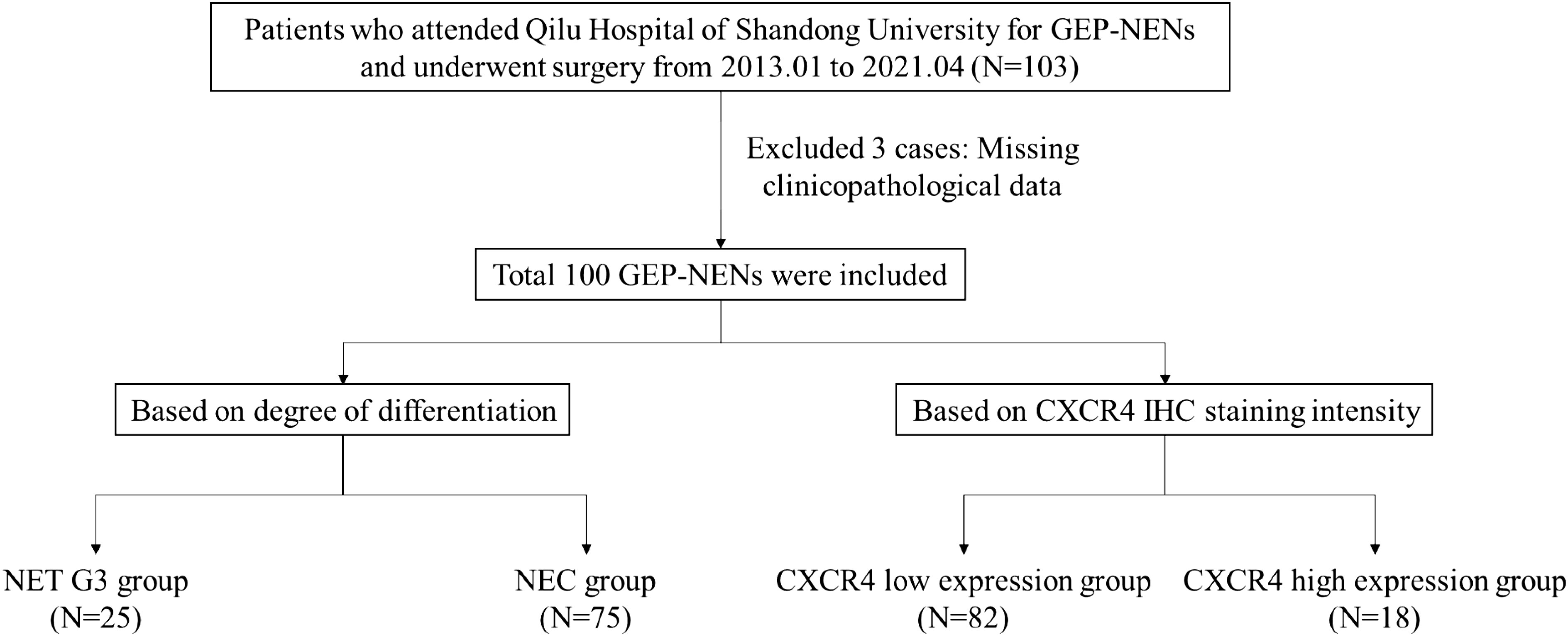
This study followed the following inclusion criteria: (1) Patients who received surgical treatment in Qilu Hospital of Shandong University. (2) Pathology confirmed that the lesion is GEP-NENs G3. (3) Patients who never received neoadjuvant therapy before surgical treatment. (4) Patients who have complete clinicopathological data. Exclusion criteria: (1) Age < 18 years old. (2) Combined with other malignant tumors. (3) Multiple neuroendocrine neoplasia, such as multiple endocrine neoplasia type 1 (MEN1), multiple endocrine neoplasia type 2 (MEN2), etc. A total of 100 participants were enrolled in this study, the flowchart of the study was shown in Figure 1.
2.3 Sample collection
Collecting serum collection before receiving treatment, the serum tumor markers, including carbohydrate antigen (CA)19-9, CA 125, carcinoembryonic antigen (CEA), alpha fetoprotein (AFP) and so on were measured after at least 12 hours of fasting. Due to the heterogeneity of NENs, we selected surgically resected specimens instead of biopsy specimens for IHC staining to ensure a more comprehensive reflection of CXCR4 expression in tumor tissues and adjacent tissues. For cases with metastatic disease, we selected only the primary tumor site because there is insufficient evidence to show the consistency of CXCR4 expression between the primary tumor and metastatic lesions.
2.4 Immunohistochemistry
The formalin-fixed paraffin-embedded tumor tissues were obtained from Department of Pathology, Qilu Hospital of Shandong University. The wax block tissue was cut into continuous tissue sections with a thickness of 4 microns by microtome, and then roasted in a drying instrument at 65°C for 2 hours until dewaxing. The sections were successively dewaxed with xylene, 100% alcohol, 90% alcohol, 80% alcohol, 70% alcohol, and finally washed three times with phosphate buffer solution (PBS). Antigen repair was performed using EDTA solution. An appropriate amount of endogenous peroxidase blocker was added to the tissue and incubated for 15 minutes at room temperature. Primary antibody Anti-CXCR4 (1:200, ab181020; EPUMBR3) was added and incubated overnight. After removing the antibody solution, use PBS to clear it three times, each time lasting 3 minutes. Drop an appropriate amount of reaction booster solution, incubate at room temperature for 20 minutes, and then wash with PBS for 3 times. After that, secondary antibody was added, and the samples were incubated at room temperature for 20 minutes, and washed with PBS for 3 times. Positive control (colon cancer tissue section) and negative control (PBS instead of primary antibody) were set for each batch.
2.5 Scoring methods
All immuno-stained sections and matched hematoxylin and eosin stained sections were scanned by optical microscopy and were evaluated by the intensity (negative scored as 0, weak scored as 1, moderate scored as 2, strong scored as 3) by two experienced pathologists. Score 0-1 was divided into CXCR4 low expression group, and score 2-3 was divided into CXCR4 high expression group.
2.6 Statistical analysis
SPSS 26.0 and GraphPad Prism 8.0 were used for data sorting and analyzing. Data were expressed as the number of cases and the rate. Continuous variables were described in terms of median and interquartile range (IQR) and were compared using the Mann-Whitney U test. Categorical variables were compared using the χ2 test. Overall survival of GEP-NEN G3 patients was estimated by using the K-M method and log-rank test. P<0.05 was considered statistically significant.
3 Results
3.1 Clinicopathological features and prognosis of GEP-NEN G3 patients
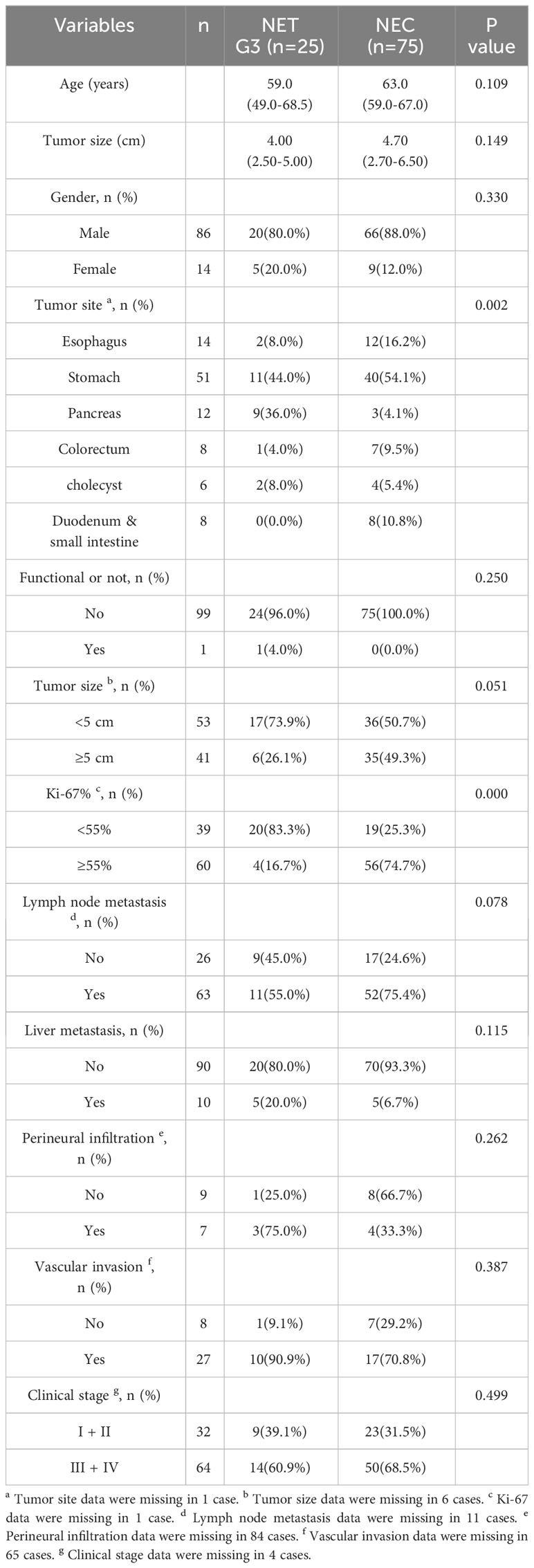
A total of 100 GEP-NENs G3 patients (25 NETs G3 and 75 NECs) were ultimately enrolled in this study. The comparison of clinicopathological characteristics between NET G3 and NEC are summarized in Table 1. The mean age of all patients was 63.1 years old, there was no significant difference in age between NET G3 and NEC (P=0.109) and in gender composition (P=0.330). The primary sites of NET G3 were stomach (11/25, 44.0%), pancreas (9/25, 36.0%), esophagus (2/25, 8.0%), gallbladder (2/25, 8.0%), and colorectal (1/25, 4.0%), in order. And the primary sites of NEC were stomach (40/75, 54.1%), esophagus (12/75, 16.2%), duodenum and small intestine (8/75, 10.8%), colorectal (7/75, 9.5%), gallbladder (4/75, 5.4%) and pancreas (3/75, 4.1%), in order. The primary site was significantly different between NET G3 and NEC (P=0.002). No matter in NEC or in NET G3, the most common primary sites were both stomachs. The maximum diameter of the tumor specimens ranged from 0.9 to 17.0 centimeters (cm), with a mean of 4.77 cm. There was no significant difference between the two groups in terms of maximum diameter of the tumor (P=0.149). The mean Ki-67 value for all specimens was 57.6%, including 39.2% for NET G3 and 65.1% for NEC. We found that among NET G3 patients, 4 cases (16.7%) have Ki-67 ≥ 55%, which significantly lower than that of patients with NEC (74.7%, 56/75) (P < 0.001). There were no significant differences between NET G3 and NEC patients in tumor function, lymph node metastasis, liver metastasis, vascular invasion, perineural infiltration, clinical stage (Table 1).
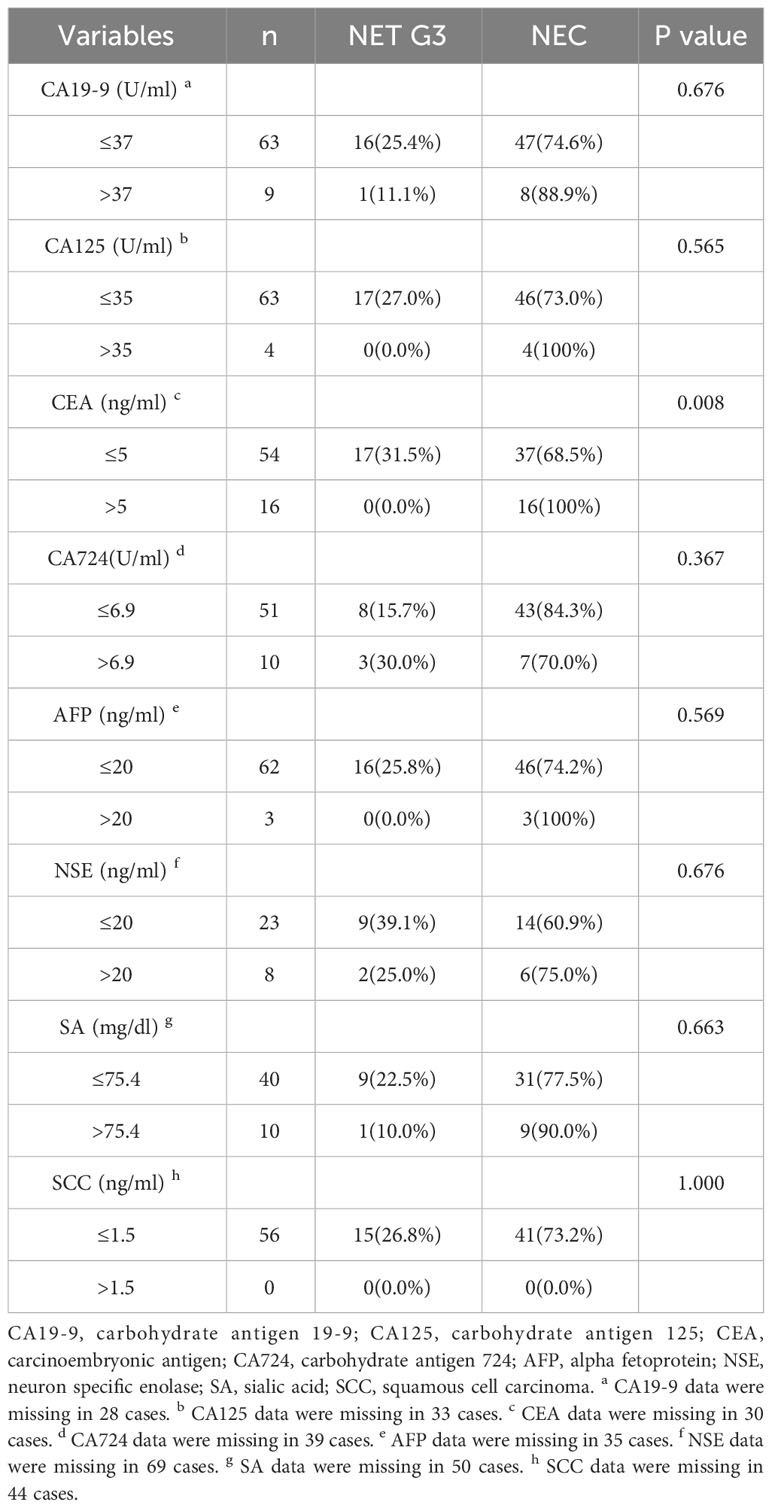
For serum tumor markers, all NET patients (17/17, 100%) have normal CEA, while only 69.8% of NEC patients (37/53) have normal CEA, and the difference between them was statistically significant (P=0.008) (Table 2).We also find that the expression level of CA125 was increased in 4 patients and the expression level of AFP was increased in 3 patients, all of whom were NEC patients, but the results were not statistically different. Furthermore, there were no significant differences in other serum tumor markers between NET G3 patients and NEC patients.
In terms of prognosis, A total of 34 patients died during the follow-up period, including 5 (14.7%) cases with NET G3 and 29 (85.3%) cases with NEC. The median OS for patients with NEC was 35.0 m (95%CI, 28.7m-41.3m), while NET G3 patients did not reach the median follow-up time by the end of the study. Log-rank test showed there was no statistical difference between the two group (P = 0.110) (Figure 2A).

Figure 2 K-M Survival curve. (A) Kaplan-Meier survival curve showed that the prognosis of NET G3 was better than NEC but there is no statistical difference between the two group (P = 0.110); (B, C) The K-M curves showed that there was no significant difference in overall survival between patients with high CXCR4 expression and patients with low CXCR4 expression, either in GEP-NEN G3 or NEC (P=0.920, P=0.842. respectively).
3.2 The expression of CXCR4 in NET G3 and NEC
CXCR4 is mainly expressed in the membrane and cytoplasm of tumor cells, and cytoplasmic coloration reflects the internalization of the receptor in response to agonist stimulation (19). Both membrane and cytoplasmic coloration were evaluated equally in this study. Among GEP-NEN G3 patients, 82 cases were classified into CXCR4 low expression group (82.0%), and 18 cases were classified into CXCR4 high expression group (18.0%). The expression of CXCR4 in the adjacent tissues was low or none, and the expression of CXCR4 was significantly different between cancer and adjacent tissues (P < 0.001). In the CXCR4 low expression group, NET G3 accounted for 29.3% (24/82) and NEC accounted for 70.7% (58/82). In the CXCR4 high expression group, 17 cases (94.4%) were NEC and only 1 case (5.6%) was NET G3. The high expression rate of CXCR4 in NEC was 5.7 times higher than that in NET G3 (17/75 vs 1/25, P=0.038). Figures 3–6 shows the expression of CXCR4 in GEP-NEN G3 of different tumor origins and in tumor and adjacent tissues.

Figure 3 Expression of CXCR4 in rectal NET G3. Immunohistochemical detection of CXCR4 expression in rectal NET G3, CXCR4 is mainly expressed in the membrane and cytoplasm of tumor cells. CXCR4 was lowly expressed in adjacent tissues (B) compared with tumor tissues (C). Original magnifications×40 (A), ×200 (B, C).

Figure 4 Expression of CXCR4 in pancreatic NET G3. Immunohistochemical detection of CXCR4 expression in pancreatic NET G3, CXCR4 is mainly expressed in the membrane and cytoplasm of tumor cells. CXCR4 was lowly expressed in adjacent tissues (B) compared with tumor tissues (C). Original magnifications×40 (A), ×200 (B, C).
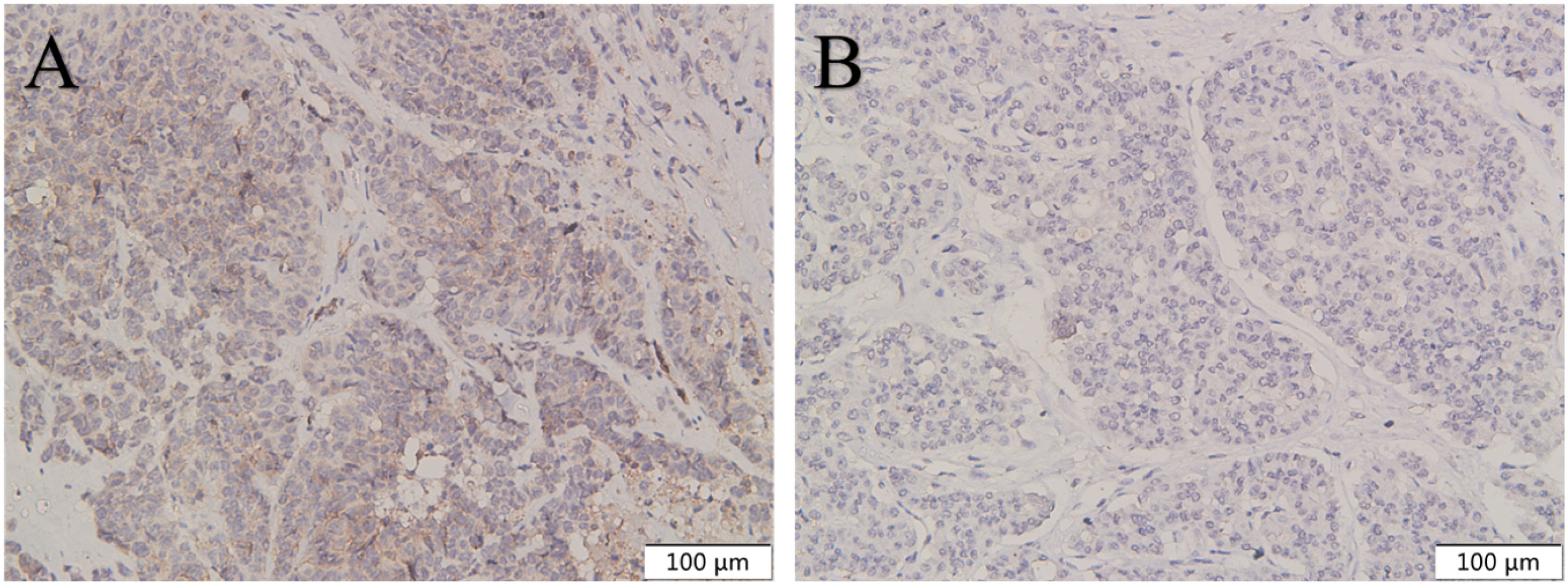
Figure 5 The expression of CXCR4 in pancreatic NEC was higher than in pancreatic NET G3. CXCR4 was highly expressed in pancreatic NEC (A) compared with pancreatic NET G3 (B). Original magnifications×100.

Figure 6 The expression of CXCR4 in gastric NEC was higher than in gastric NET G3. CXCR4 was highly expressed in gastric NEC (A) compared with gastric NET G3 (B). Original magnifications×100.
3.3 Relationship between the expression level of CXCR4 and clinicopathological features in NEC and GEP-NEN G3
The expression level of CXCR4 in NEC patients with different tumor origins was significantly different (P=0.013). The proportion of CXCR4 overexpression was the highest in pancreatic NEC patients (66.7%), followed by colorectal (57.1%), gallbladder (50.0%), esophagus (25.0%) and stomach (15.0%). The expression level of CXCR4 in NEC patients was not significantly correlated with gender, tumor size and other clinicopathological parameters (Table 3).
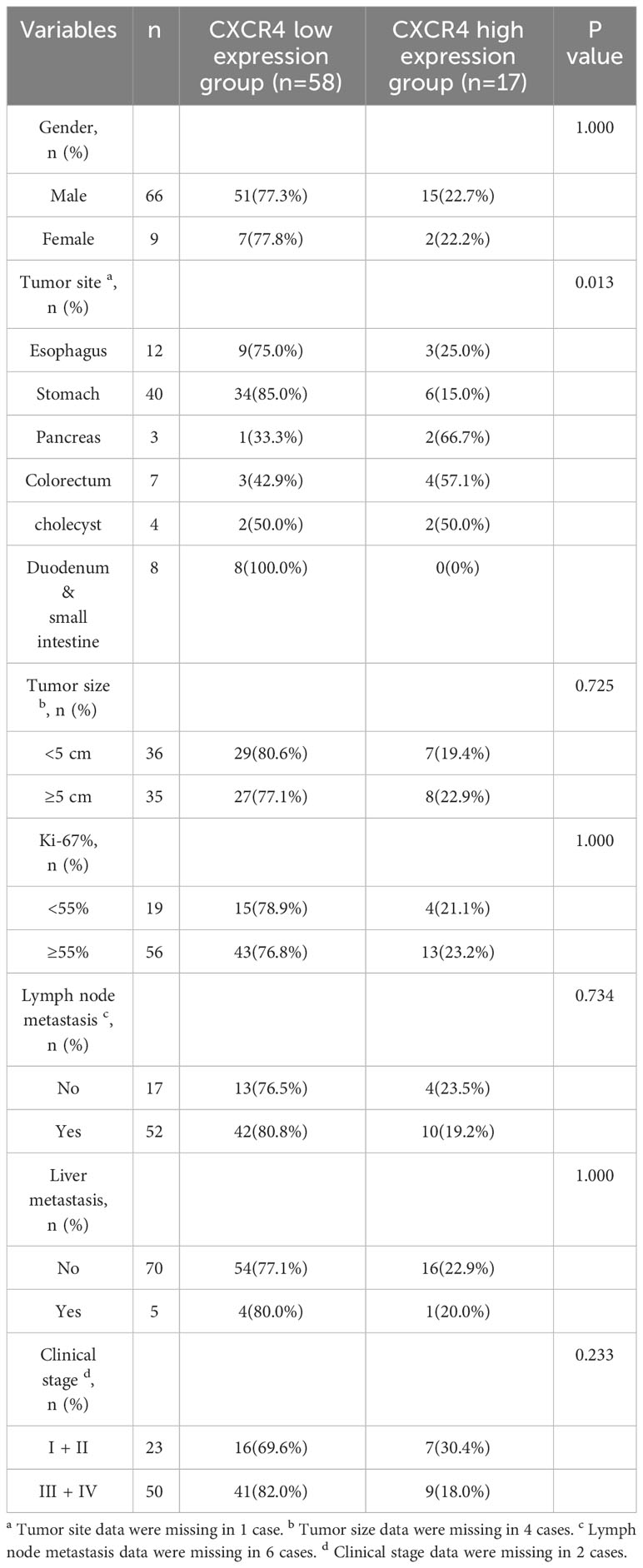
Table 3 Relationship between the expression level of CXCR4 and clinicopathological features in NEC patients.
We further explore the relationship between CXCR4 expression and clinicopathological features in patients with GEP-NEN G3 (Table 4). However, there is no statistically significant difference in the expression level of CXCR4 between tumors of different primary sites (P = 0.057). We find that the high expression rate of CXCR4 in patients with Ki67 index ≥ 55% was higher than that in patients with Ki67 index < 55%, although the difference was not statistically significant (21.6% vs 12.8%, P = 0.265). The expression level of CXCR4 was not significantly correlated with gender, lymph node metastasis, liver metastasis and clinical stage (Table 4). The K-M curves showed that there was no significant difference in OS between patients with high CXCR4 expression and patients with low CXCR4 expression, either in GEP-NEN G3 or NEC (P=0.920, P=0. 842. respectively) (Figures 2B, C).

Table 4 Relationship between the expression level CXCR4 and clinicopathological features in GEP-NEN G3 patients.
4 Discussion
The origin, molecular mechanism, biological behavior, histopathological features, clinical manifestations, treatment and prognosis of NET G3 are different from those of NEC (2, 20). This study systematically analyzed the clinicopathological features and prognosis and investigated the expression of CXCR4 in tumor and adjacent tissues of patients with GEP-NENs G3 who underwent surgical treatment in Qilu Hospital of Shandong University. We hope our study can provide reference for further related research and clinical practice.
A total of 100 patients were enrolled in this study, focusing on the relationship between CXCR4 and GEP-NENs G3, in order to better distinguish NET G3 and NEC. We found that tumor primary location (P=0.002), and Ki-67 index (P<0.001) were significantly different between NET G3 and NEC. The expression level of CXCR4 in GEP-NENs G3 tumors and adjacent tissues was significantly different (P<0.001). In the CXCR4 low expression group, NET G3 accounted for 29.3% (24 cases) and NEC accounted for 70.7% (58 cases). In the CXCR4 high expression group, 17 cases (94.4%) were NEC and only 1 case (5.6%) was NET G3. And the high expression rate of CXCR4 in NEC was 5.7 times higher than that in NET G3 (22.7% vs 4%, P=0.038).
Considering that the distinction between NET G3 and NEC relies mainly on proliferation index and cell differentiation. Studies have explored diagnostic markers to distinguish NET G3 from NEC. Liverani et al. found that DLL3 is expressed in poorly differentiated NEC but not in NET G3 and is associated with poor prognosis. They concluded that DLL3 is considered to serve as a NEC diagnostic marker and potential therapeutic target (21). Bremer et al. similarly used immunohistochemistry to find that high expression of Enhancer of Zeste Homolog 2 (EZH2) in high-grade NENs was associated with poorer OS and was also significantly correlated with histological manifestations of NEC, proposing that EZH2 could be used as a biomarker to differentiate NET G3 from NEC (22). Therefore, effective markers to distinguish NET G3 from NEC are expected to be promising.
Muller et al. first explored the expression of CXCR4 in tumors in 2001, CXCR4 was highly expressed in breast cancer, but not expressed or low-expressed in normal breast tissues (23). Joseph Kim et al. also found that 89% of malignant melanoma patients with liver metastases and 97% of colorectal cancer patients with liver metastases expressed CXCR4. IHC staining of melanoma liver metastases and colorectal cancer liver metastases showed CXCR4 cytomembrane and cytoplasm staining pattern, while normal hepatocytes showed absent or weak staining (24). And several previous studies have explored CXCR4 expression in neuroendocrine tumors, but these studies generally took GEP-NENs as a whole rather than focusing on GEP-NENs G3, and the number of specimens included in these studies was also generally small (25–27). This study reveals that in GEP-NENs G3, the expression level of CXCR4 in tumors and adjacent tissues was significantly different (P<0.001) and in NEC was significantly higher than that in NET G3 (P=0.038).
Previous studies have confirmed that high expression of CXCR4 can promote tumor growth and metastasis (28, 29). In this study, the proportion of CXCR4 high expression in GEP-NENs G3 tumor diameter ≥5cm group was higher than that in tumor diameter < 5cm group, but the difference was not statistically significant (19.5% vs 15.1%, P = 0.572). Compared with the group without lymph node or liver metastasis, the proportion of CXCR4 high expression in the group with metastasis was slightly higher, but there was no statistical significance. R Marechal et al. found that the high expression of CXCR4 is an independent risk factor for poor prognosis of pancreatic cancer (30). In other cancer, there are also many studies confirmed that high expression of CXCR4 is associated with poor prognosis of patients (31, 32). However, in our study, we found that the prognosis of patients was not related to CXCR4 expression level, on matter in GEP-NENs G3 or NEC.
Epidemiological investigation showed that the common sites of GEP-NENs in western countries were small intestine, rectum, pancreas and stomach, while the common sites of NENs in China were pancreas, rectum and stomach (33). In this study, stomach was the most common primary site of GEP-NENs G3 (51.0%), followed by esophagus, pancreas and colorectal. However, only GEP-NENs G3 patients who received surgery in Qilu Hospital of Shandong University were included, which has selection biases and could not represent the overall epidemiological characteristics of them.
The Ki-67 index of NEC is usually significantly higher than that of NET G3 (34). Some studies have pointed out that the optimal cut-off value of Ki-67 for distinguishing pNET G3 from pNEC is 55% (35). Elettra Merola et al. found that Ki-67 index ≥55% is an independent risk factor for poor prognosis, and radical surgery is an effective treatment for resectable GEP-NENs G3 patients, especially for patients with Ki-67 index < 55% (36). The current study found that the proportion of Ki-67≥55% in NEC was significantly higher than that in NET G3 (P<0.01), and the proliferation activity of NEC was significantly higher than that of NET G3. And we also found that NEC patients had a significantly higher proportion of CEA abnormalities than NET G3 patients. All of these indicators indicate that NEC is more aggressive and malignant.
This study suggested that high CXCR4 expression was more likely to lead to a diagnosis of NEC, CXCR4 can be used in the diagnosis of high-grade neuroendocrine tumors. However, there are some limitations of this study. Firstly, we are a single-center retrospective study, and only patients who underwent surgery were included, which may result in selection bias, the sample size is small, especially the patients with NET G3, and contains specimens of many different tissues such as stomach, pancreas. Secondly, the follow-up time of this study is short, some patients were not observed to the occurrence of terminal events, the number of lost visits accounted for a high percentage, which caused a great deal of trouble for prognostic related research. What’s more, this study retrospectively collected histological specimens and performed IHC staining, however the staining quality of IHC was low and the CXCR4 staining scores had an impact. So, multi-center clinical studies with larger sample size and longer follow-up time are needed to further explore the clinicopathological characteristics, prognosis and reasonable treatment of GEP-NEN G3, and to further confirm the relationship between CXCR4 expression level and clinicopathological features and prognosis of GEP-NEN G3.
5 Conclusions
We found that the tumor location, and Ki-67 index were significantly different between NET G3 and NEC. CXCR4 was highly expressed only in tumor tissues, and low or no expression was found in the adjacent tissues. And the expression of CXCR4 in NEC was significantly higher than that in NET G3. However, we found there’s no significant association between CXCR4 expression level and patient prognosis.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
This study has passed the review of the ethics Committee of Qilu Hospital of Shandong University (Approval number: QLCR20220462). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
CP: Writing – review & editing, Conceptualization. YL: Writing – review & editing, Software, Writing – original draft. MS: Writing – original draft, Data curation, Investigation, Methodology. ZF: Investigation, Methodology, Formal analysis, Supervision, Writing – review & editing. XG: Investigation, Writing – review & editing, Validation. YM: Investigation, Writing – review & editing, Software. SL: Writing – review & editing, Formal analysis, Methodology. CG: Writing – review & editing, Conceptualization, Data curation. PS: Writing – review & editing, Methodology, Resources, Visualization. XW: Writing – review & editing, Methodology, Resources, Validation, Visualization. HZ: Writing – review & editing, Funding acquisition, Project administration, Resources, Supervision.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China (81972274, 81702365), Taishan Scholars Program for Young Expert of Shandong Province (tsqn202103172). Shandong Provincial Natural Science Foundation (ZR2021LSW004, ZR2017MH090), Clinical Research Foundation of Shandong University (2020SDUCRCC016).
Acknowledgments
The authors thank all colleagues who contributed to this effort.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
CXCR4, CXC chemokine receptor 4; GEP-NENs G3, Gastroenteropancreatic Neuroendocrine Neoplasms Grade 3; IHC, Immunohistochemistry; K-M, Kaplan-Meier; CEA, Carcinoembryonic antigen; NET, Neuroendocrine tumor; NEC, Neuroendocrine carcinoma; CA 19-9, Carbohydrate antigen; AFP, Alpha fetoprotein; PBS, phosphate buffer solution; IQR, Interquartile range.
References
1. Cives M, Strosberg JR. Gastroenteropancreatic neuroendocrine tumors. CA Cancer J Clin. (2018) 68:471–87. doi: 10.3322/caac.21493
2. Shi M, Fan Z, Xu J, Yang J, Li Y, Gao C, et al. Gastroenteropancreatic neuroendocrine neoplasms G3: Novel insights and unmet needs. Biochim Biophys Acta Rev Cancer. (2021) 1876:188637. doi: 10.1016/j.bbcan.2021.188637
3. Lawrence B, Gustafsson BI, Chan A, Svejda B, Kidd M, Modlin IM. The epidemiology of gastroenteropancreatic neuroendocrine tumors. Endocrinol Metab Clin North Am. (2011) 40:1–18. doi: 10.1016/j.ecl.2010.12.005
4. Dasari A, Shen C, Halperin D, Zhao B, Zhou S, Xu Y, et al. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol. (2017) 3:1335–42. doi: 10.1001/jamaoncol.2017.0589
5. Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, et al. The 2019 WHO classification of tumours of the digestive system. Histopathology. (2020) 76:182–8. doi: 10.1111/his.13975
6. Pavel M, Oberg K, Falconi M, Krenning EP, Sundin A, Perren A, et al. Gastroenteropancreatic neuroendocrine neoplasms: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. (2020) 31:844–60. doi: 10.1016/j.annonc.2020.03.304
7. Yachida S, Vakiani E, White CM, Zhong Y, Saunders T, Morgan R, et al. Small cell and large cell neuroendocrine carcinomas of the pancreas are genetically similar and distinct from well-differentiated pancreatic neuroendocrine tumors. Am J Surg Pathol. (2012) 36:173–84. doi: 10.1097/PAS.0b013e3182417d36
8. Marinoni I, Kurrer AS, Vassella E, Dettmer M, Rudolph T, Banz V, et al. Loss of DAXX and ATRX are associated with chromosome instability and reduced survival of patients with pancreatic neuroendocrine tumors. Gastroenterology. (2014) 146:453–60 e5. doi: 10.1053/j.gastro.2013.10.020
9. Xing J, Chen J, You T, Sun Z, Lu T, Cheng Y, et al. Expression of p53 and Rb reveal subtypes of gastric neuroendocrine carcinoma with distinct prognosis. J Neuroendocrinol. (2023) 35:e13257. doi: 10.1111/jne.13257
10. Vaday GG, Lider O. Extracellular matrix moieties, cytokines, and enzymes: dynamic effects on immune cell behavior and inflammation. J Leukoc Biol. (2000) 67:149–59. doi: 10.1002/jlb.67.2.149
11. Veldkamp CT, Ziarek JJ, Su J, Basnet H, Lennertz R, Weiner JJ, et al. Monomeric structure of the cardioprotective chemokine SDF-1/CXCL12. Protein Sci. (2009) 18:1359–69. doi: 10.1002/pro.167
12. Guembarovski AL, Guembarovski RL, Hirata BKB, Vitiello GAF, Suzuki KM, Enokida MT, et al. CXCL12 chemokine and CXCR4 receptor: association with susceptibility and prognostic markers in triple negative breast cancer. Mol Biol Rep. (2018) 45:741–50. doi: 10.1007/s11033-018-4215-7
13. Bai S, Wang D, Klein MJ, Siegal GP. Characterization of CXCR4 expression in chondrosarcoma of bone. Arch Pathol Lab Med. (2011) 135:753–8. doi: 10.1043/2009-0230-OA.110.5858/2009-0230-OA.1
14. Zhao BC, Wang ZJ, Mao WZ, Ma HC, Han JG, Zhao B, et al. CXCR4/SDF-1 axis is involved in lymph node metastasis of gastric carcinoma. World J Gastroenterol. (2011) 17:2389–96. doi: 10.3748/wjg.v17.i19.2389
15. Wang Y, Zhao L, Han X, Wang Y, Mi J, Wang C, et al. Saikosaponin A inhibits triple-negative breast cancer growth and metastasis through downregulation of CXCR4. Front Oncol. (2019) 9:1487. doi: 10.3389/fonc.2019.01487
16. Yin X, Liu Z, Zhu P, Wang Y, Ren Q, Chen H, et al. CXCL12/CXCR4 promotes proliferation, migration, and invasion of adamantinomatous craniopharyngiomas via PI3K/AKT signal pathway. J Cell Biochem. (2019) 120:9724–36. doi: 10.1002/jcb.28253
17. Circelli L, Sciammarella C, Guadagno E, Tafuto S, del Basso de Caro M, Botti G, et al. CXCR4/CXCL12/CXCR7 axis is functional in neuroendocrine tumors and signals on mTOR. Oncotarget. (2016) 7:18865–75. doi: 10.18632/oncotarget.7738
18. Popa O, Taban SM, Dema ALC, Plopeanu AD, Barna RA, Cornianu M, et al. Immunohistochemical expression of chemokine receptor in neuroendocrine neoplasms (CXCR4) of the gastrointestinal tract: a retrospective study of 71 cases. Rom J Morphol Embryol. (2021) 62:151–7. doi: 10.47162/RJME.62.1.14
19. Mai R, Kaemmerer D, Trager T, Neubauer E, Sanger J, Baum RP, et al. Different somatostatin and CXCR4 chemokine receptor expression in gastroenteropancreatic neuroendocrine neoplasms depending on their origin. Sci Rep. (2019) 9:4339. doi: 10.1038/s41598-019-39607-2
20. Venizelos A, Elvebakken H, Perren A, Nikolaienko O, Deng W, Lothe IMB, et al. The molecular characteristics of high-grade gastroenteropancreatic neuroendocrine neoplasms. Endocr Relat Cancer. (2021) 29:1–14. doi: 10.1530/ERC-21-0152
21. Liverani C, Bongiovanni A, Mercatali L, Pieri F, Spadazzi C, Miserocchi G, et al. Diagnostic and predictive role of DLL3 expression in gastroenteropancreatic neuroendocrine neoplasms. Endocr Pathol. (2021) 32:309–17. doi: 10.1007/s12022-020-09657-8
22. Bremer SCB, Bittner G, Elakad O, Dinter H, Gaedcke J, Konig AO, et al. Enhancer of zeste homolog 2 (EZH2) is a marker of high-grade neuroendocrine neoplasia in gastroenteropancreatic and pulmonary tract and predicts poor prognosis. Cancers (Basel). (2022) 14(12):2828. doi: 10.3390/cancers14122828
23. Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. (2001) 410:50–6. doi: 10.1038/35065016
24. Kim J, Mori T, Chen SL, Amersi FF, Martinez SR, Kuo C, et al. Chemokine receptor CXCR4 expression in patients with melanoma and colorectal cancer liver metastases and the association with disease outcome. Ann Surg. (2006) 244:113–20. doi: 10.1097/01.sla.0000217690.65909.9c
25. Deschamps L, Bacha D, Rebours V, Mebarki M, Bretagnol F, Panis Y, et al. The expression of the hypoxia markers CA9 and CXCR4 is correlated with survival in patients with neuroendocrine tumours of the ileum. Neuroendocrinology. (2012) 95:214–22. doi: 10.1159/000329873
26. Kaemmerer D, Trager T, Hoffmeister M, Sipos B, Hommann M, Sanger J, et al. Inverse expression of somatostatin and CXCR4 chemokine receptors in gastroenteropancreatic neuroendocrine neoplasms of different Malignancy. Oncotarget. (2015) 6:27566–79. doi: 10.18632/oncotarget.4491
27. Refardt J, Hofland J, Kwadwo A, Nicolas GP, Rottenburger C, Fani M, et al. Theranostics in neuroendocrine tumors: an overview of current approaches and future challenges. Rev Endocr Metab Disord. (2021) 22:581–94. doi: 10.1007/s11154-020-09552-x
28. Yang L, Jackson E, Woerner BM, Perry A, Piwnica-Worms D, Rubin JB. Blocking CXCR4-mediated cyclic AMP suppression inhibits brain tumor growth in vivo. Cancer Res. (2007) 67:651–8. doi: 10.1158/0008-5472.CAN-06-2762
29. Darash-Yahana M, Pikarsky E, Abramovitch R, Zeira E, Pal B, Karplus R, et al. Role of high expression levels of CXCR4 in tumor growth, vascularization, and metastasis. FASEB J. (2004) 18:1240–2. doi: 10.1096/fj.03-0935fje
30. Marechal R, Demetter P, Nagy N, Berton A, Decaestecker C, Polus M, et al. High expression of CXCR4 may predict poor survival in resected pancreatic adenocarcinoma. Br J Cancer. (2009) 100:1444–51. doi: 10.1038/sj.bjc.6605020
31. Scala S, Ottaiano A, Ascierto PA, Cavalli M, Simeone E, Giuliano P, et al. Expression of CXCR4 predicts poor prognosis in patients with Malignant melanoma. Clin Cancer Res. (2005) 11:1835–41. doi: 10.1158/1078-0432.CCR-04-1887
32. Kaifi JT, Yekebas EF, Schurr P, Obonyo D, Wachowiak R, Busch P, et al. Tumor-cell homing to lymph nodes and bone marrow and CXCR4 expression in esophageal cancer. J Natl Cancer Inst. (2005) 97:1840–7. doi: 10.1093/jnci/dji431
33. Fan JH, Zhang YQ, Shi SS, Chen YJ, Yuan XH, Jiang LM, et al. A nation-wide retrospective epidemiological study of gastroenteropancreatic neuroendocrine neoplasms in China. Oncotarget. (2017) 8:71699–708. doi: 10.18632/oncotarget.17599
34. Busico A, Maisonneuve P, Prinzi N, Pusceddu S, Centonze G, Garzone G, et al. Gastroenteropancreatic high-grade neuroendocrine neoplasms: histology and molecular analysis, two sides of the same coin. Neuroendocrinology. (2020) 110:616–29. doi: 10.1159/000503722
35. Shi H, Chen L, Zhang Q, Lin Y, Jiang C, Yao H, et al. Concordance between the ki-67 index cutoff value of 55% and differentiation in neuroendocrine tumor and neuroendocrine carcinoma in grade 3 pancreatic neuroendocrine neoplasms. Pancreas. (2020) 49:1378–82. doi: 10.1097/MPA.0000000000001693
Keywords: GEP-NEN G3, neuroendocrine neoplasm, CXCR4, clinicopathological features, prognosis
Citation: Pang C, Li Y, Shi M, Fan Z, Gao X, Meng Y, Liu S, Gao C, Su P, Wang X and Zhan H (2024) Expression and clinical value of CXCR4 in high grade gastroenteropancreatic neuroendocrine neoplasms. Front. Endocrinol. 15:1281622. doi: 10.3389/fendo.2024.1281622
Received: 22 August 2023; Accepted: 23 February 2024;
Published: 08 March 2024.
Edited by:
Godfrey Grech, University of Malta, MaltaReviewed by:
Alberto Bongiovanni, IRCCS Istituto Romagnolo per lo Studio dei Tumori (IRST) “Dino Amadori”, ItalyIsmaheel Lawal, Emory University, United States
Copyright © 2024 Pang, Li, Shi, Fan, Gao, Meng, Liu, Gao, Su, Wang and Zhan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hanxiang Zhan, zhanhanxiang@hotmail.com; Xiao Wang, wangxiao08@sdu.edu.cn
†These authors have contributed equally to this work and share first authorship
 Chaoyu Pang1†
Chaoyu Pang1† Yongzheng Li
Yongzheng Li Zhiyao Fan
Zhiyao Fan Xin Gao
Xin Gao Changhao Gao
Changhao Gao Hanxiang Zhan
Hanxiang Zhan