Abstract
Background
Glutathione peroxidase 2 (GPX2) is an antioxidant enzyme with an important role in tumor progression in various cancers. However, the clinical significance of GPX2 in lung adenocarcinoma has not been clarified.
Methods
Quantitative reverse transcription polymerase chain reaction (qRT-PCR) was used to analyze GPX2 mRNA expression. Then, we conducted immunohistochemistry (IHC) to assess GPX2 expression in specimens acquired from 351 patients with lung adenocarcinoma who underwent surgery at Kyushu University from 2003 to 2012. We investigated the association between GPX2 expression and clinicopathological characteristics and further analyzed the prognostic relevance.
Results
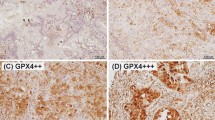
qRT-PCR revealed that GPX2 mRNA expression was notably higher in tumor cells than in normal tissues. IHC revealed that high GPX2 expression (n = 175, 49.9%) was significantly correlated with male sex, smoking, advanced pathological stage, and the presence of pleural, lymphatic, and vascular invasion. Patients with high GPX2 expression exhibited significantly shorter recurrence-free survival (RFS) and overall survival. Multivariate analysis identified high GPX2 expression as an independent prognostic factor of RFS.
Conclusions
GPX2 expression was significantly associated with pathological malignancy. It is conceivable that high GPX2 expression reflects tumor malignancy. Therefore, high GPX2 expression is a significant prognostic factor of poor prognosis for completely resected lung adenocarcinoma.




Similar content being viewed by others
References
Siegel RL, Miller KD, Wagle NS, et al. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17–48.
Ramalingam SS, Vansteenkiste J, Planchard D, et al. Overall survival with Osimertinib in untreated, EGFR-mutated advanced NSCLC. N Engl J Med. 2020;382(1):41–50.
Recondo G, Facchinetti F, Olaussen KA, et al. Making the first move in EGFR-driven or ALK-driven NSCLC: first-generation or next-generation TKI? Nat Rev Clin Oncol. 2018;15(11):694–708.
Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823–33.
Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389(10066):255–65.
Sahoo BM, Banik BK, Borah P, et al. Reactive oxygen species (ROS): Key components in cancer therapies. Anticancer Agents Med Chem. 2022;22(2):215–22.
Prasad S, Gupta SC, Tyagi AK. Reactive oxygen species (ROS) and cancer: role of antioxidative nutraceuticals. Cancer Lett. 2017;387:95–105.
Redza-Dutordoir M, Averill-Bates DA. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim Biophys Acta. 2016;1863(12):2977–92.
Ishimoto T, Nagano O, Yae T, et al. CD44 variant regulates redox status in cancer cells by stabilizing the xCT subunit of system xc(-) and thereby promotes tumor growth. Cancer Cell. 2011;19(3):387–400.
Kumari S, Badana AK, Malla R. Reactive oxygen species: a key constituent in cancer survival. Biomarker Insights. 2018;6(13):1177271918755391.
Griess B, Tom E, Domann F, et al. Extracellular superoxide dismutase and its role in cancer. Free Radic Biol Med. 2017;112:464–79.
Pei J, Pan X, Wei G, et al. Research progress of glutathione peroxidase family (GPX) in redoxidation. Front Pharmacol. 2023;14:1147414.
Singh A, Rangasamy T, Thimmulappa RK, et al. Glutathione peroxidase 2, the major cigarette smoke-inducible isoform of GPX in lungs, is regulated by Nrf2. Am J Respir Cell Mol Biol. 2006;35(6):639–50.
Zalewska-Ziob M, Adamek B, Kasperczyk J, et al. Activity of antioxidant enzymes in the tumor and adjacent noncancerous tissues of non-small-cell lung cancer. Oxid Med Cell Longev. 2019;2019:2901840.
Esworthy RS, Doroshow JH, Chu FF. The beginning of GPX2 and 30 years later. Free Radic Biol Med. 2022;188:419–33.
Du H, Chen B, Jiao NL, et al. Elevated glutathione peroxidase 2 expression promotes cisplatin resistance in lung adenocarcinoma. Oxid Med Cell Longev. 2020;2020:7370157.
Peng F, Xu Q, Jing X, et al. GPX2 promotes EMT and metastasis in non-small cell lung cancer by activating PI3K/AKT/mTOR/Snail signaling axis. FASEB Bioadv. 2023;5(6):233–50.
Liu T, Kan XF, Ma C, et al. GPX2 overexpression indicates poor prognosis in patients with hepatocellular carcinoma. Tumour Biol. 2017;39(6):1010428317700410.
Yang H, Wang W, Zhang Y, et al. The role of NF-E2-related factor 2 in predicting chemoresistance and prognosis in advanced non-small-cell lung cancer. Clin Lung Cancer. 2011;12(3):166–71.
Zimta AA, Cenariu D, Irimie A, Magdo L, Nabavi SM, Atanasov AG, Berindan-Neagoe I. The role of Nrf2 activity in cancer development and progression. Cancers. 2019;11(11):1755.
Liu D, Sun L, Tong J, et al. Prognostic significance of glutathione peroxidase 2 in gastric carcinoma. Tumour Biol. 2017;39(6):1010428317701443.
Naiki T, Naiki-Ito A, Asamoto M, et al. GPX2 overexpression is involved in cell proliferation and prognosis of castration-resistant prostate cancer. Carcinogenesis. 2014;35(9):1962–7.
Ren Z, Liang H, Galbo PM Jr, et al. Redox signaling by glutathione peroxidase 2 links vascular modulation to metabolic plasticity of breast cancer. Proceed Nat Acad Sci. 2022;119(8):e2107266119.
Lei Z, Tian D, Zhang C, et al. Clinicopathological and prognostic significance of GPX2 protein expression in esophageal squamous cell carcinoma. BMC Cancer. 2016;16:410.
Li F, Dai L, Niu J. GPX2 silencing relieves epithelial-mesenchymal transition, invasion, and metastasis in pancreatic cancer by downregulating Wnt pathway. J Cell Physiol. 2020;235(11):7780–90.
Xu H, Hu C, Wang Y, et al. Glutathione peroxidase 2 knockdown suppresses gastric cancer progression and metastasis via regulation of kynurenine metabolism. Oncogene. 2023;42(24):1994–2006.
Satoh H, Moriguchi T, Saigusa D, et al. NRF2 intensifies host defense systems to prevent lung carcinogenesis, but after tumor initiation accelerates malignant cell growth. Cancer Res. 2016;76(10):3088–96.
Huang H, Zhang W, Pan Y, et al. YAP suppresses lung squamous cell carcinoma progression via deregulation of the DNp63-GPX2 axis and ROS accumulation. Cancer Res. 2017;77(21):5769–81.
Wang M, Chen X, Fu G, et al. Glutathione peroxidase 2 overexpression promotes malignant progression and cisplatin resistance of KRAS-mutated lung cancer cells. Oncol Rep. 2022;48(6):1–4.
Ahmed KM, Veeramachaneni R, Deng D, et al. Glutathione peroxidase 2 is a metabolic driver of the tumor immune microenvironment and immune checkpoint inhibitor response. J Immunother Cancer. (2022);10(8)
Acknowledgment
We thank Joe Barber, Jr., PhD, from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
All authors declare no conflicts of interest associated with this research. The graphical abstract has been designed using freely available resources from Flaticon.com.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10434_2024_15116_MOESM1_ESM.pdf
Nrf2 expression in lung adenocarcinoma specimens. Examples of Nrf2 staining with intensity scores of (a) 0, (b) 1, and (c) 2. Scale bar: 200 μm. Nrf2, nuclear factor erythroid 2-related factor 2; IS, intensity score (PDF 78 kb)
10434_2024_15116_MOESM2_ESM.pdf
The correlation between GPX2 and NFE2L2 expression in lung adenocarcinoma as determined by GSEA using a dataset from TCGA (a). Nrf2 expression in patients with surgically resected lung adenocarcinoma and high (n = 30) or low GPX2 (n = 30) expression on IHC analysis (b). GPX2, glutathione peroxidase 2; GSEA, gene set enrichment analysis; IHC, immunohistochemistry; Nrf2, nuclear factor erythroid 2-related factor 2; TCGA, The Cancer Genome Atlas (PDF 77 kb)
10434_2024_15116_MOESM3_ESM.pdf
GPX2 expression in normal lung tissue and lung adenocarcinoma tissue in TCGA analysis (a). Kaplan–Meier curves of overall survival in patients with surgically resected lung adenocarcinoma according to GPX2 expression in TCGA analysis (b). GPX2, glutathione peroxidase 2; TCGA, The Cancer Genome Atlas (PDF 53 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hashinokuchi, A., Matsubara, T., Ono, Y. et al. Clinical and Prognostic Significance of Glutathione Peroxidase 2 in Lung Adenocarcinoma. Ann Surg Oncol (2024). https://doi.org/10.1245/s10434-024-15116-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1245/s10434-024-15116-z