miR-132 inhibits lung cancer cell migration and invasion by targeting SOX4
Introduction
MicroRNAs (miRNAs) are a class of small non-coding RNAs that negatively regulate gene expression at post-transcription level by target mRNAs degradation or translation repression. Mounting evidence has suggested that deregulation of miRNAs expression is often implicated in variety of disorders associating with kinds of human disease such as metabolic disorders, cardiovascular disease, and particular cancer (1-4). In addition, increasing evidence also indicated miRNAs could be key players in tumor initiation and progression and affected tumor cell invasion and metastasis (5-7).
miR-132, arising from the miR-212/132 cluster, located in the intron of a non-coding gene on chromosome 17 in humans (8). Studies have shown that the miR-132 is involved in the vascular smooth muscle dysfunction mediated by angiotensin II (Ang-II) (9). In the tumorigenesis, it is reported that downregulation of miR-132 prohibits proliferation, invasion, migration and metastasis in breast cancer by targeting HN1 (10). Additionally, miR-132 inhibits colorectal cancer invasion and metastasis via targeting ZEB2 (11). However, the mechanism of miR-132 regulated tumor metastasis is still need to be further explored.
The SOX4 (sex-determining region Y-box 4) gene, a member of the SOX family, has been shown to have important roles in the development and cell fate decision. In tumorgenesis, increasing evidence suggests that SOX4 was significantly elevated in multiple human cancers, including breast cancer, prostate cancer, liver cancer and lung cancer (12-14). Its overexpression was closely correlated with tumor progression and metastasis (15,16). Besides, SOX4 was shown to regulate several key signaling pathways in cancer cells. For instance, SOX4 could regulate β-catenin/T-cell factor activity and act as an agonist of Wnt signaling in colon cancer (17).
In the present study, we found that miR-132 was significantly down-regulated in lung cancer cells, and further revealed that the overexpression of miR-132 could inhibit lung cancer progression in vitro and in vivo. Additionally, we identified that SOX4 is a target gene of miR-132. miR-132 is able to inhibit invasion of lung cancer cells by paralyzing the function of SOX4.
Materials and methods
Cell culture and transfection
The human lung adenocarcinoma cell line A549, Lung squamous carcinoma cell line YTMLC-9, lung large cell carcinoma cell line H460 and normal human bronchial epithelial (HBE) cells (obtained from Tianjin Key Laboratory of Lung Cancer Metastasis and Tumor Microenvironment, Tianjin Lung Cancer Institute, China) were cultured in RMPI 1640 medium containing 10% fetal bovine serum (FBS) (Gibco, CA, USA) at 37 °C with 5% CO2 incubator. For transfection, cells were cultured to 80% confluence and transfected with recombinant eukaryotic vector and empty vector using Lipofectamine 2000 (Invitrogen, CA, USA) according to the manufacturer’s recommendation.
Quantitative real-time PCR (qRT-PCR)
qRT-PCR was performed to validate the miRNA expression level. qRT-PCR was carried out using SYBR®Premix Ex TaqTM (Takara, Japan). PCR were carried out in triplicate and analyzed using the ABI Prism 7900HT fast RT-PCR system (Applied Biosystems, Life technologies, USA). The relative quantification value for each gene was calculated by the 2-ΔΔCt method using U6 or GAPDH as an internal reference. All primers were shown in Table S1.

Full table
Plasmid constructions
The full-length 3’-untranslated region (3’UTR) of SOX4 was amplified from human genomic DNA, and was cloned into the downstream of the firefly luciferase coding region of pMIR-GLOTM Luciferase vector (Promega, USA). The recombined vector was named as pMIR-SOX4. Mutations of miR-132 binding sites were introduced by site-directed mutagenesis and the resulted vector was named pMIR-SOX4-Mut. Primers used for the constructions were listed in Table S1. All the constructions were confirmed by sequencing.
Cell migration assay
The migration ability was determined using wound-healing assay. The cells were plated into 12-well plates without antibiotics; cells were transfected with miR-132 mimic or mimic control. Twenty-four h later, transfected cells were wounded with a sterile plastic 100 µL micropipette tip, the floating debris were washed with PBS and cultured in serum-free medium. Width of the wound was measured at different time points. Three to four different locations were visualized and photographed under a phase-contrast inverted microscope.
Cell invasion assay
Boyden chamber assay was used to examine cell invasion capability. A549 cells were transfected with miR-132 mimic or mimic control. Sixteen h later, transfected cells were trypsinized and resuspended, 1.0×104 cells in 200 µL RPMI 1640 medium were placed into the upper chambers (8-mm pore size; Millipore). The lower chambers were filled with 600 µL complete medium with 10% FBS. After incubation for 12 h at 37 °C, non-invading cells were removed from the top of the chamber with a cotton swab. The invasion cells on the lower surface of the inserts were fixed and stained with 0.1% crystal violet, and five random fields for each insert were counted at 200× magnifications.
In vivo assay
For the in vivo assays, 5×105 A549 cells stably expressing miR-132 or negative control (NC) were injected subcutaneously to the mouse. The mice were observed over 4 weeks for tumor formation. After the mice were sacrificed, the tumors were recovered and the weight of each tumor was determined.
Dual-luciferase reporter assay
Cells were seeded into 24-well plates and cotransfected with 200 ng of pMIR-SOX4 or pMIR-SOX4-Mut vector and 100 ng of miR-132 mimic or mimic control, and the pRL-TK plasmid (Promega, Madison, WI) which was used as internal normalization. After 48 h, cells were lysed using the lysis buffer (Promega). Luciferase reporter gene assay was implemented using the Dual-Luciferase Reporter Assay System (Promega) according to the manufacturer’s instructions. All experiments were performed at least 3 times.
Western blotting
Cells were transfected with either miR-132 or pCMV-Tag-2b-SOX4. Total cell extracts prepared from cells using RIPA buffer (Beyotime, China), were resolved on 10% gradient SDS-polacrylamide gel and transferred NC membranes. Membranes were blocked for 1 h in 5% skim milk in TBST and incubated with anti-SOX4 antibody (1:1,000, Santa Cruz) or anti-β-actin antibody (1:5,000, CST). Overnight at 4 °C, followed by the incubation with appropriate HRP-conjugated secondary antibody at optimized concentration. The densitometry of Western blot results was measured using ImageJ software.
Statistical analysis
The data were presented as mean ± standard deviation (SD). T-test was used to determine the significant differences between control and treatment groups. Statistical analysis was performed using SPSS15.0 software and P<0.05 was considered to be a statistically significant difference.
Results
miR-132 is down-regulated in non-small cell lung cancer (NSCLC) cells
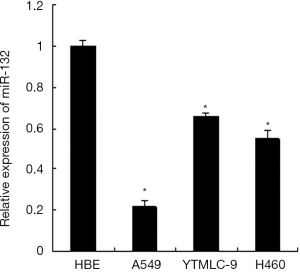
We analyzed the expression of miR-132 in human normal bronchial epithelial cells HBE and three human lung cancer cell lines including A549, YTMLC-9 and H460 by qRT-PCR. The results showed that miR-132 was down-regulated significantly in all the three lung cancer cell lines compared with the HBE cell line (Figure 1). These data indicate that the reduced expression of miR-132 is a frequent event in NSCLC cells, which may be involved in lung cancer progression.
miR-132 overexpression inhibits the aggressiveness of NSCLC cells in vitro
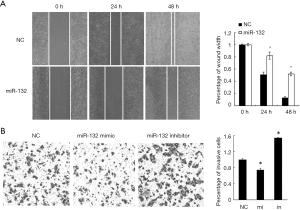
Next, we tested the role of miR-132 in NSCLC cells, its mimics or NCs were transfected into A549 cells. Wound healing assay showed that the ectopic expression of miR-132 in A549 cells significantly inhibited cell migration, compared to the control group (Figure 2A). In agreement, Boyden chamber assay revealed that miR-132 overexpressing cells showed decreased invasion ability, compared to NC cells (Figure 2B). Taken together, our results indicate that miR-132 is able to suppress the migration and invasion of NSCLC cells in vitro.
miR-132 overexpression suppresses NSCLC progression in vivo
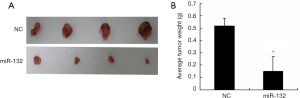
To further determine the roles of miR-132, A549 cells with stable overexpression of miR-132 were generated and injected subcutaneously to the nude mice. The tumor growth was closely monitored for another 4 weeks. As a result, the tumor size (Figure 3A) and weight (Figure 3B) were markedly reduced in miR-132-overexpressed group, compared to control group, suggesting that miR-132 could also suppress NSCLC progression in vivo.
miR-132 directly targets SOX4 in NSCLC cells
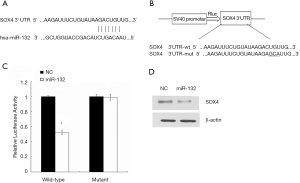
To detect the molecular mechanism by which miR-132 suppresses the metastasis of lung cancer cells, we predicted the putative target genes of miR-132 in human cells using the tool miRanda, PicTar and TargetScans. Among which, we found that the gene encoding SOX4 harbored a potential miR-132 binding site (Figure 4A). Therefore, the wild type or mutant 3’UTR of SOX4 gene was cloned and inserted into pMIR reporter vector (Figure 4B). Overexpression of miR-132 led to a reduction of luciferase activity carrying the wild-type 3’UTR. However, mutation of the potential miR-132 binding site abolished the inhibitory roles of miR-132 (Figure 4C). Moreover, transfection of miR-132 mimics in lung cancer cells resulted in a reduced SOX4 expression1Z1 (Figure 4D). Therefore, our results suggest that SOX4 might be a target of miR-132 in NSCLC cells.
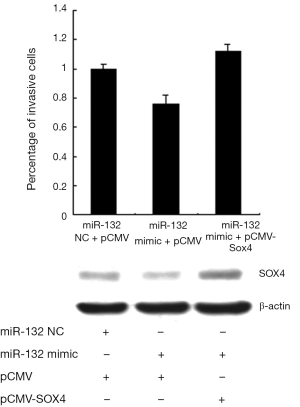
SOX4 contributes to miR-132 suppressed invasion of NSCLC cells
To further verify the functional effect of SOX4 on miR-132-mediated regulation of invasion. A549 cells were transfected with SOX4 expression plasmid after transfection of miR-132 mimics. As shown in Figure 5, transfection of miR-132 mimic into A549 cells led to a decrease of cell invasion, whereas SOX4 re-introduction reversed the anti-invasion role of miR-132, underlining the specific importance of the SOX4 for miR-132 action in the cell invasion. Additionally, the overexpression efficiency of SOX4 was examined by Western.
Discussion
Advances in diagnostic techniques and therapeutic means have improved the early detection and reduced the mortality rate of lung cancer; however, it still is the first leading cause of cancer-related deaths worldwide and is responsible for more than 1 million deaths every year. The primary reason for its mortality and relapse is that lung cancer cells have a powerful ability to metastasis. Although the great progresses has been made in recent decades and it has been suggested that the tumor metastasis is a complex process including multiple sequential steps, the molecular mechanisms that regulate metastasis in lung cancer cells are still poorly understood.
In our study, we found that miR-132 was markedly decreased in lung cancer cells. Ectopic expression miR-132 was able to inhibit migration and invasion and of NSCLC cells in vitro. Furthermore, the anti-tumorigenesis role of miR-132 was performed in vivo. The data show that miR-132 may be a tumor suppressor in the development and progression of lung cancer. This result is similar with our previous report (18). However, the mechanism of miR-132 regulated migration and invasion of NSCLC still need to be further investigated.
At the molecular level, Zhao et al. found that miR-132 could suppress the G1/S phase transition of the cell cycle and the epithelial to mesenchymal transition in cervical cancer cells by targeting SMAD2 (19). Lei et al. found that miR-132 inhibits proliferation of hepatic carcinoma cells by targeting YAP (20). Additionally, You et al. found that miR-132 could suppress the migration and invasion of lung cancer cells via targeting the EMT regulator ZEB2 (18). Our present study found that SOX4 was a functional target of miR-132 by luciferase reporter gene assays and western blot analysis method. SOX4 is a transcription factor required for tissue development and differentiation in vertebrates (21). Recently, overexpression of SOX4 has been reported in many cancers including NSCLC (15,22-25). Overexpression of SOX4 was correlated with poor prognosis in patients with NSCLC. Zhou et al. demonstrated that SOX4 could regulate lung cancer cell metastasis (26). So we thought that SOX4 may be involved in miR-132 mediated lung cancer cell metastasis. In our study, we found that SOX4 could reverse the anti-invasion role of miR-132.
In summary, we investigated the role of miR-132 in NSCLC development. Our finding suggests that miR-132 may be a novel tumor suppressor miRNA. miR-132 blocks the migration and invasion of NSCLC cells through targeting SOX4. Our data provide new insight into the mechanism responsible for the development of human NSCLC. Additionally, miR-132 may serve as a potential therapeutic candidate in the treatment of NSCLC.
Acknowledgements
Funding: This study was partly supported by the grants from National Natural Science Foundation of China (No. 81201852), National 863 Program (No. 2012AA02A201, No. 2012AA02A502), and National 973 Program (No. 2010CB529405)
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Kalyani A, Sonawane PJ, Khan AA, et al. Post-Transcriptional Regulation of Renalase Gene by miR-29 and miR-146 MicroRNAs: Implications for Cardiometabolic Disorders. J Mol Biol 2015;427:2629-46. [PubMed]
- Vatandoost N, Amini M, Iraj B, et al. Dysregulated miR-103 and miR-143 expression in peripheral blood mononuclear cells from induced prediabetes and type 2 diabetes rats. Gene 2015;572:95-100. [PubMed]
- Zhao W, Zhao SP, Zhao YH. MicroRNA-143/-145 in Cardiovascular Diseases. Biomed Res Int 2015;2015:531740.
- Chen L, Min L, Wang X, et al. Loss of RACK1 Promotes Metastasis of Gastric Cancer by Inducing a miR-302c/IL8 Signaling Loop. Cancer Res 2015;75:3832-41. [PubMed]
- Cui R, Meng W, Sun HL, et al. MicroRNA-224 promotes tumor progression in nonsmall cell lung cancer. Proc Natl Acad Sci U S A 2015;112:E4288-97. [PubMed]
- Sheng L, He P, Yang X, et al. miR-612 negatively regulates colorectal cancer growth and metastasis by targeting AKT2. Cell Death Dis 2015;6:e1808.
- Hanniford D, Zhong J, Koetz L, et al. A miRNA-based signature detected in primary melanoma tissue predicts development of brain metastasis. Clin Cancer Res 2015. [Epub ahead of print]. [PubMed]
- Lau P, Bossers K, Janky R, et al. Alteration of the microRNA network during the progression of Alzheimer's disease. EMBO Mol Med 2013;5:1613-34. [PubMed]
- Jin W, Reddy MA, Chen Z, et al. Small RNA sequencing reveals microRNAs that modulate angiotensin II effects in vascular smooth muscle cells. J Biol Chem 2012;287:15672-83. [PubMed]
- Zhang ZG, Chen WX, Wu YH, et al. miR-132 prohibits proliferation, invasion, migration, and metastasis in breast cancer by targeting HN1. Biochem Biophys Res Commun 2014;454:109-14. [PubMed]
- Zheng YB, Luo HP, Shi Q, et al. miR-132 inhibits colorectal cancer invasion and metastasis via directly targeting ZEB2. World J Gastroenterol 2014;20:6515-22. [PubMed]
- Castillo SD, Matheu A, Mariani N, et al. Novel transcriptional targets of the SRY-HMG box transcription factor SOX4 link its expression to the development of small cell lung cancer. Cancer Res 2012;72:176-86. [PubMed]
- Zhang J, Liang Q, Lei Y, et al. SOX4 induces epithelial-mesenchymal transition and contributes to breast cancer progression. Cancer Res 2012;72:4597-608. [PubMed]
- Wang L, Li Y, Yang X, et al. ERG-SOX4 interaction promotes epithelial-mesenchymal transition in prostate cancer cells. Prostate 2014;74:647-58. [PubMed]
- Wang L, Zhang J, Yang X, et al. SOX4 is associated with poor prognosis in prostate cancer and promotes epithelial-mesenchymal transition in vitro. Prostate Cancer Prostatic Dis 2013;16:301-7. [PubMed]
- Zhang H, Alberich-Jorda M, Amabile G, et al. Sox4 is a key oncogenic target in C/EBPα mutant acute myeloid leukemia. Cancer Cell 2013;24:575-88. [PubMed]
- Lee AK, Ahn SG, Yoon JH, et al. Sox4 stimulates ß-catenin activity through induction of CK2. Oncol Rep 2011;25:559-65. [PubMed]
- You J, Li Y, Fang N, et al. miR-132 suppresses the migration and invasion of lung cancer cells via targeting the EMT regulator ZEB2. PLoS One 2014;9:e91827. [PubMed]
- Zhao JL, Zhang L, Guo X, et al. miR-212/132 downregulates SMAD2 expression to suppress the G1/S phase transition of the cell cycle and the epithelial to mesenchymal transition in cervical cancer cells. IUBMB Life 2015;67:380-94. [PubMed]
- Lei CJ, Li L, Gao X, et al. Hsa-miR-132 inhibits proliferation of hepatic carcinoma cells by targeting YAP. Cell Biochem Funct 2015;33:326-33. [PubMed]
- Potzner MR, Tsarovina K, Binder E, et al. Sequential requirement of Sox4 and Sox11 during development of the sympathetic nervous system. Development 2010;137:775-84. [PubMed]
- Wang D, Hao T, Pan Y, et al. Increased expression of SOX4 is a biomarker for malignant status and poor prognosis in patients with non-small cell lung cancer. Mol Cell Biochem 2015;402:75-82. [PubMed]
- Wang W, Zhang J, Zhan X, et al. SOX4 is associated with poor prognosis in cholangiocarcinoma. Biochem Biophys Res Commun 2014;452:614-21. [PubMed]
- Jafarnejad SM, Ardekani GS, Ghaffari M, et al. Sox4-mediated Dicer expression is critical for suppression of melanoma cell invasion. Oncogene 2013;32:2131-9. [PubMed]
- Wang C, Zhao H, Lu J, et al. Clinicopathological significance of SOX4 expression in primary gallbladder carcinoma. Diagn Pathol 2012;7:41. [PubMed]
- Zhou Y, Wang X, Huang Y, et al. Down-regulated SOX4 expression suppresses cell proliferation, metastasis and induces apoptosis in Xuanwei female lung cancer patients. J Cell Biochem 2015;116:1007-18. [PubMed]


