Abstract
Background and Aims
Nonalcoholic fatty liver disease (NAFLD), currently referred to as metabolic dysfunction-associated steatotic liver disease (MASLD), affects approximately 38% of the world’s population, yet no pharmacological therapies have been approved for treatment. We conducted a traditional and network meta-analysis to comprehensively assess the effectiveness of drug regimens on NAFLD, and continued to use the old terminology for consistency.
Methods
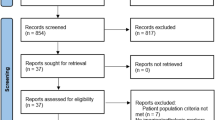
Randomized, placebo-controlled trials (RCTs) investigating drug therapy in an adult population diagnosed with NAFLD with or without diabetes mellitus were included. We assessed the quality of RCTs via the Risk of Bias 2 (ROB 2) tool. When I2 < 50%, we chose a random-effects model, otherwise a fixed-effects model was selected. A random effects model was applied in the network meta-analysis. The odds ratio (OR), weighted mean difference (WMD) or standard mean difference (SMD) with 95% confidence interval (CI) were used for outcome evaluation. The primary endpoint was the resolution of nonalcoholic steatohepatitis (NASH) without the worsening of liver fibrosis. Other endpoints included histological findings and metabolic changes. The PROSPERO Registration ID was CRD42023404309.
Results
Thiazolidinediones (TZDs), vitamin E plus pioglitazone, glucagon-like peptide-1 (GLP-1) receptor agonists and fibroblast growth factor-21 (FGF-21) analogue had a higher surface under the cumulative ranking curve (SUCRA = 76.6, 73.0, 72.0 and 71.6) regarding NASH resolution. Improvement of liver fibrosis stage (≥ 1) was observed with obeticholic acid 25 mg/day (OR 2.01, 95% CI 1.35–2.98), lanifibranor 1200 mg/day (OR 2.39, 95% CI 1.19–4.82) and silymarin (OR 4.54, 95% CI 1.18–17.43) in traditional meta-analysis.
Conclusions
The results of the comprehensive analysis suggested hypoglycemic drug therapy as an effective intervention for NAFLD, with or without diabetes mellitus. A prioritized selection of TZDs, vitamin E plus pioglitazone, GLP-1 receptor agonists and FGF-21 analogue may be considered for NASH resolution. Obeticholic acid, lanifibranor and silymarin could be considered for the improvement of liver fibrosis. Each medication was relatively safe compared with placebo.



Similar content being viewed by others
References
Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease—meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73–84.
Wong VW, Ekstedt M, Wong GL, Hagström H. Changing epidemiology, global trends and implications for outcomes of NAFLD. J Hepatol. 2023;79(3):842–52.
Rinella ME, Lazarus JV, Ratziu V, Francque SM, Sanyal AJ, Kanwal F, Romero D, Abdelmalek MF, Anstee QM, Arab JP, et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. J Hepatol. 2023;79(6):1542–56.
Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, George J, Bugianesi E. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15(1):11–20.
Friedman SL, Neuschwander-Tetri BA, Rinella M, Sanyal AJ. Mechanisms of NAFLD development and therapeutic strategies. Nat Med. 2018;24(7):908–22.
Stefan N, Cusi K. A global view of the interplay between non-alcoholic fatty liver disease and diabetes. Lancet Diabetes Endocrinol. 2022;10(4):284–96.
Shi J, Luo D, Weng H, Zeng XT, Lin L, Chu H, Tong T. Optimally estimating the sample standard deviation from the five-number summary. Res Synth Methods. 2020;11(5):641–54.
Higgins JPT, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane handbook for systematic reviews of interventions. Chichester: Wiley; 2019.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372: n71.
Harrer M, Cuijpers P, Furukawa TA, Ebert DD. Doing meta-analysis with R: a hands-on guide. 1st ed. Boca Raton: Chapman & Hall/CRC Press; 2021.
Newsome PN, Buchholtz K, Cusi K, Linder M, Okanoue T, Ratziu V, Sanyal AJ, Sejling AS, Harrison SA. A placebo-controlled trial of subcutaneous semaglutide in nonalcoholic steatohepatitis. N Engl J Med. 2021;384(12):1113–24.
Flint A, Andersen G, Hockings P, Johansson L, Morsing A, Sundby Palle M, Vogl T, Loomba R, Plum-Mörschel L. Randomised clinical trial: semaglutide versus placebo reduced liver steatosis but not liver stiffness in subjects with non-alcoholic fatty liver disease assessed by magnetic resonance imaging. Aliment Pharmacol Ther. 2021;54(9):1150–61.
Armstrong MJ, Gaunt P, Aithal GP, Barton D, Hull D, Parker R, Hazlehurst JM, Guo K, Abouda G, Aldersley MA, et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. 2016;387(10019):679–90.
Ratziu V, Giral P, Jacqueminet S, Charlotte F, Hartemann-Heurtier A, Serfaty L, Podevin P, Lacorte JM, Bernhardt C, Bruckert E, et al. Rosiglitazone for nonalcoholic steatohepatitis: one-year results of the randomized placebo-controlled Fatty Liver Improvement with Rosiglitazone Therapy (FLIRT) Trial. Gastroenterology. 2008;135(1):100–10.
Aithal GP, Thomas JA, Kaye PV, Lawson A, Ryder SD, Spendlove I, Austin AS, Freeman JG, Morgan L, Webber J. Randomized, placebo-controlled trial of pioglitazone in nondiabetic subjects with nonalcoholic steatohepatitis. Gastroenterology. 2008;135(4):1176–84.
Balas B, Belfort R, Harrison SA, Darland C, Finch J, Schenker S, Gastaldelli A, Cusi K. Pioglitazone treatment increases whole body fat but not total body water in patients with non-alcoholic steatohepatitis. J Hepatol. 2007;47(4):565–70.
Belfort R, Harrison SA, Brown K, Darland C, Finch J, Hardies J, Balas B, Gastaldelli A, Tio F, Pulcini J, et al. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med. 2006;355(22):2297–307.
Huang JF, Dai CY, Huang CF, Tsai PC, Yeh ML, Hsu PY, Huang SF, Bair MJ, Hou NJ, Huang CI, et al. First-in-Asian double-blind randomized trial to assess the efficacy and safety of insulin sensitizer in nonalcoholic steatohepatitis patients. Hepatol Int. 2021;15(5):1136–47.
Cusi K, Orsak B, Bril F, Lomonaco R, Hecht J, Ortiz-Lopez C, Tio F, Hardies J, Darland C, Musi N, et al. Long-term pioglitazone treatment for patients with nonalcoholic steatohepatitis and prediabetes or type 2 diabetes mellitus: a randomized trial. Ann Intern Med. 2016;165(5):305–15.
Taheri H, Malek M, Ismail-Beigi F, Zamani F, Sohrabi M, Reza Babaei M, Khamseh ME. Effect of empagliflozin on liver steatosis and fibrosis in patients with non-alcoholic fatty liver disease without diabetes: a randomized, double-blind, placebo-controlled trial. Adv Ther. 2020;37(11):4697–708.
Joy TR, McKenzie CA, Tirona RG, Summers K, Seney S, Chakrabarti S, Malhotra N, Beaton MD. Sitagliptin in patients with non-alcoholic steatohepatitis: a randomized, placebo-controlled trial. World J Gastroenterol. 2017;23(1):141–50.
Cui J, Philo L, Nguyen P, Hofflich H, Hernandez C, Bettencourt R, Richards L, Salotti J, Bhatt A, Hooker J, et al. Sitagliptin vs. placebo for non-alcoholic fatty liver disease: a randomized controlled trial. J Hepatol. 2016;65(2):369–76.
Younossi ZM, Ratziu V, Loomba R, Rinella M, Anstee QM, Goodman Z, Bedossa P, Geier A, Beckebaum S, Newsome PN, et al. Obeticholic acid for the treatment of non-alcoholic steatohepatitis: interim analysis from a multicentre, randomised, placebo-controlled phase 3 trial. Lancet. 2019;394(10215):2184–96.
Patel K, Harrison SA, Elkhashab M, Trotter JF, Herring R, Rojter SE, Kayali Z, Wong VW, Greenbloom S, Jayakumar S, et al. Cilofexor, a nonsteroidal FXR agonist, in patients with noncirrhotic NASH: a phase 2 randomized controlled trial. Hepatology. 2020;72(1):58–71.
Ratziu V, de Ledinghen V, Oberti F, Mathurin P, Wartelle-Bladou C, Renou C, Sogni P, Maynard M, Larrey D, Serfaty L, et al. A randomized controlled trial of high-dose ursodesoxycholic acid for nonalcoholic steatohepatitis. J Hepatol. 2011;54(5):1011–9.
Leuschner UF, Lindenthal B, Herrmann G, Arnold JC, Rössle M, Cordes HJ, Zeuzem S, Hein J, Berg T. High-dose ursodeoxycholic acid therapy for nonalcoholic steatohepatitis: a double-blind, randomized, placebo-controlled trial. Hepatology. 2010;52(2):472–9.
Lindor KD, Kowdley KV, Heathcote EJ, Harrison ME, Jorgensen R, Angulo P, Lymp JF, Burgart L, Colin P. Ursodeoxycholic acid for treatment of nonalcoholic steatohepatitis: results of a randomized trial. Hepatology. 2004;39(3):770–8.
Šmíd V, Dvořák K, Šedivý P, Kosek V, Leníček M, Dezortová M, Hajšlová J, Hájek M, Vítek L, Bechyňská K, et al. Effect of omega-3 polyunsaturated fatty acids on lipid metabolism in patients with metabolic syndrome and NAFLD. Hepatol Commun. 2022;6(6):1336–49.
Cansanção K, Citelli M, Carvalho Leite N, López de Las Hazas MC, Dávalos A, Tavares do Carmo MDG, Peres WAF. Impact of long-term supplementation with fish oil in individuals with non-alcoholic fatty liver disease: a double blind randomized placebo controlled clinical trial. Nutrients. 2020;12(11):3372.
Scorletti E. Effects of purified eicosapentaenoic and docosahexaenoic acids in nonalcoholic fatty liver disease: results from the WELCOME study. Hepatology. 2014;60:1211–21.
Tobin D, Brevik-Andersen M, Qin Y, Innes JK, Calder PC. Evaluation of a high concentrate omega-3 for correcting the omega-3 fatty acid nutritional deficiency in non-alcoholic fatty liver disease (CONDIN). Nutrients. 2018;10(8):1126.
Okada L, Oliveira CP, Stefano JT, Nogueira MA, Silva I, Cordeiro FB, Alves VAF, Torrinhas RS, Carrilho FJ, Puri P, et al. Omega-3 PUFA modulate lipogenesis, ER stress, and mitochondrial dysfunction markers in NASH—proteomic and lipidomic insight. Clin Nutr. 2018;37(5):1474–84.
Dasarathy S, Dasarathy J, Khiyami A, Yerian L, Hawkins C, Sargent R, McCullough AJ. Double-blind randomized placebo-controlled clinical trial of omega 3 fatty acids for the treatment of diabetic patients with nonalcoholic steatohepatitis. J Clin Gastroenterol. 2015;49(2):137–44.
Argo CK, Patrie JT, Lackner C, Henry TD, de Lange EE, Weltman AL, Shah NL, Al-Osaimi AM, Pramoonjago P, Jayakumar S, et al. Effects of n-3 fish oil on metabolic and histological parameters in NASH: a double-blind, randomized, placebo-controlled trial. J Hepatol. 2015;62(1):190–7.
Zhu FS, Liu S, Chen XM, Huang ZG, Zhang DW. Effects of n-3 polyunsaturated fatty acids from seal oils on nonalcoholic fatty liver disease associated with hyperlipidemia. World J Gastroenterol. 2008;14(41):6395–400.
Sanyal AJ, Abdelmalek MF, Suzuki A, Cummings OW, Chojkier M. No significant effects of ethyl-eicosapentanoic acid on histologic features of nonalcoholic steatohepatitis in a phase 2 trial. Gastroenterology. 2014;147(2):377-384.e371.
Nelson A, Torres DM, Morgan AE, Fincke C, Harrison SA. A pilot study using simvastatin in the treatment of nonalcoholic steatohepatitis: a randomized placebo-controlled trial. J Clin Gastroenterol. 2009;43(10):990–4.
Merat S, Malekzadeh R, Sohrabi MR, Sotoudeh M, Rakhshani N, Sohrabpour AA, Naserimoghadam S. Probucol in the treatment of non-alcoholic steatohepatitis: a double-blind randomized controlled study. J Hepatol. 2003;38(4):414–8.
Zelber-Sagi S, Kessler A, Brazowsky E, Webb M, Lurie Y, Santo M, Leshno M, Blendis L, Halpern Z, Oren R. A double-blind randomized placebo-controlled trial of orlistat for the treatment of nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2006;4(5):639–44.
Le TA, Chen J, Changchien C, Peterson MR, Kono Y, Patton H, Cohen BL, Brenner D, Sirlin C, Loomba R. Effect of colesevelam on liver fat quantified by magnetic resonance in nonalcoholic steatohepatitis: a randomized controlled trial. Hepatology. 2012;56(3):922–32.
Ratziu V, Harrison SA, Francque S, Bedossa P, Lehert P, Serfaty L, Romero-Gomez M, Boursier J, Abdelmalek M, Caldwell S, et al. Elafibranor, an agonist of the peroxisome proliferator-activated receptor-α and -δ, induces resolution of nonalcoholic steatohepatitis without fibrosis worsening. Gastroenterology. 2016;150(5):1147-1159.e1145.
Nakajima A, Eguchi Y, Yoneda M, Imajo K, Tamaki N, Suganami H, Nojima T, Tanigawa R, Iizuka M, Iida Y, et al. Randomised clinical trial: pemafibrate, a novel selective peroxisome proliferator-activated receptor α modulator (SPPARMα), versus placebo in patients with non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2021;54(10):1263–77.
Francque SM, Bedossa P, Ratziu V, Anstee QM, Bugianesi E, Sanyal AJ, Loomba R, Harrison SA, Balabanska R, Mateva L, et al. A randomized, controlled trial of the pan-PPAR agonist lanifibranor in NASH. N Engl J Med. 2021;385(17):1547–58.
Lukenda Zanko V, Domislovic V, Trkulja V, Krznaric-Zrnic I, Turk-Wensveen T, Krznaric Z, Filipec Kanizaj T, Radic-Kristo D, Bilic-Zulle L, Orlic L, et al. Vitamin D for treatment of non-alcoholic fatty liver disease detected by transient elastography: a randomized, double-blind, placebo-controlled trial. Diabetes Obes Metab. 2020;22(11):2097–106.
Geier A, Eichinger M, Stirnimann G, Semela D, Tay F, Seifert B, Tschopp O, Bantel H, Jahn D, Marques Maggio E, et al. Treatment of non-alcoholic steatohepatitis patients with vitamin D: a double-blinded, randomized, placebo-controlled pilot study. Scand J Gastroenterol. 2018;53(9):1114–20.
Barchetta I, Del Ben M, Angelico F, Di Martino M, Fraioli A, La Torre G, Saulle R, Perri L, Morini S, Tiberti C, et al. No effects of oral vitamin D supplementation on non-alcoholic fatty liver disease in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled trial. BMC Med. 2016;14:92.
Pervez MA, Khan DA, Slehria AUR, Ijaz A. Delta-tocotrienol supplementation improves biochemical markers of hepatocellular injury and steatosis in patients with nonalcoholic fatty liver disease: a randomized, placebo-controlled trial. Complement Ther Med. 2020;52: 102494.
Kheong CW, Mustapha NRN, Mahadeva S. A randomized trial of silymarin for the treatment of nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol. 2017;15(12):1940-1949.e1948.
Navarro VJ, Belle SH, D’Amato M, Adfhal N, Brunt EM, Fried MW, Reddy KR, Wahed AS, Harrison S. Silymarin in non-cirrhotics with non-alcoholic steatohepatitis: a randomized, double-blind, placebo controlled trial. PLoS ONE. 2019;14(9): e0221683.
Harrison SA, Abdelmalek MF, Neff G, Gunn N, Guy CD, Alkhouri N, Bashir MR, Freilich B, Kohli A, Khazanchi A, et al. Aldafermin in patients with non-alcoholic steatohepatitis (ALPINE 2/3): a randomised, double-blind, placebo-controlled, phase 2b trial. Lancet Gastroenterol Hepatol. 2022;7(7):603–16.
Harrison SA, Neff G, Guy CD, Bashir MR, Paredes AH, Frias JP, Younes Z, Trotter JF, Gunn NT, Moussa SE, et al. Efficacy and safety of aldafermin, an engineered FGF19 analog, in a randomized, double-blind, placebo-controlled trial of patients with nonalcoholic steatohepatitis. Gastroenterology. 2021;160(1):219-231.e211.
Guo W, Tian W, Lin L, Xu X. Liraglutide or insulin glargine treatments improves hepatic fat in obese patients with type 2 diabetes and nonalcoholic fatty liver disease in twenty-six weeks: a randomized placebo-controlled trial. Diabetes Res Clin Pract. 2020;170: 108487.
Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, Neuschwander-Tetri BA, Lavine JE, Tonascia J, Unalp A, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362(18):1675–85.
Bril F, Biernacki DM, Kalavalapalli S, Lomonaco R, Subbarayan SK, Lai J, Tio F, Suman A, Orsak BK, Hecht J, et al. Role of vitamin E for nonalcoholic steatohepatitis in patients with type 2 diabetes: a randomized controlled trial. Diabetes Care. 2019;42(8):1481–8.
Balmer ML, Siegrist K, Zimmermann A, Dufour JF. Effects of ursodeoxycholic acid in combination with vitamin E on adipokines and apoptosis in patients with nonalcoholic steatohepatitis. Liver Int. 2009;29(8):1184–8.
Loguercio C, Andreone P, Brisc C, Brisc MC, Bugianesi E, Chiaramonte M, Cursaro C, Danila M, de Sio I, Floreani A, et al. Silybin combined with phosphatidylcholine and vitamin E in patients with nonalcoholic fatty liver disease: a randomized controlled trial. Free Radic Biol Med. 2012;52(9):1658–65.
Harrison SA, Torgerson S, Hayashi P, Ward J, Schenker S. Vitamin E and vitamin C treatment improves fibrosis in patients with nonalcoholic steatohepatitis. Am J Gastroenterol. 2003;98(11):2485–90.
Abdelmalek MF, Sanyal AJ, Nakajima A, Neuschwander-Tetri BA, Goodman ZD, Lawitz EJ, Harrison SA, Jacobson IM, Imajo K, Gunn N, et al. Pegbelfermin in patients with nonalcoholic steatohepatitis and compensated cirrhosis (FALCON 2): a randomized phase 2b study. Clin Gastroenterol Hepatol. 2024;22(1):113-123.e119.
Loomba R, Sanyal AJ, Nakajima A, Neuschwander-Tetri BA, Goodman ZD, Harrison SA, Lawitz EJ, Gunn N, Imajo K, Ravendhran N, et al. Pegbelfermin in patients with nonalcoholic steatohepatitis and stage 3 fibrosis (FALCON 1): a randomized phase 2b study. Clin Gastroenterol Hepatol. 2024;22(1):102-112.e109.
Harrison SA, Bedossa P, Guy CD, Schattenberg JM, Loomba R, Taub R, Labriola D, Moussa SE, Neff GW, Rinella ME, et al. A phase 3, randomized, controlled trial of resmetirom in NASH with liver fibrosis. N Engl J Med. 2024;390(6):497–509.
Loomba R, Sanyal AJ, Kowdley KV, Bhatt DL, Alkhouri N, Frias JP, Bedossa P, Harrison SA, Lazas D, Barish R, et al. Randomized, controlled trial of the FGF21 analogue pegozafermin in NASH. N Engl J Med. 2023;389(11):998–1008.
Harrison SA, Frias JP, Neff G, Abrams GA, Lucas KJ, Sanchez W, Gogia S, Sheikh MY, Behling C, Bedossa P, et al. Safety and efficacy of once-weekly efruxifermin versus placebo in non-alcoholic steatohepatitis (HARMONY): a multicentre, randomised, double-blind, placebo-controlled, phase 2b trial. Lancet Gastroenterol Hepatol. 2023;8(12):1080–93.
Loomba R, Abdelmalek MF, Armstrong MJ, Jara M, Kjær MS, Krarup N, Lawitz E, Ratziu V, Sanyal AJ, Schattenberg JM, et al. Semaglutide 2·4 mg once weekly in patients with non-alcoholic steatohepatitis-related cirrhosis: a randomised, placebo-controlled phase 2 trial. Lancet Gastroenterol Hepatol. 2023;8(6):511–22.
Zewinger S, Drechsler C, Kleber ME, Dressel A, Riffel J, Triem S, Lehmann M, Kopecky C, Säemann MD, Lepper PM, et al. Serum amyloid A: high-density lipoproteins interaction and cardiovascular risk. Eur Heart J. 2015;36(43):3007–16.
Harrison SA, Taub R, Neff GW, Lucas KJ, Labriola D, Moussa SE, Alkhouri N, Bashir MR. Resmetirom for nonalcoholic fatty liver disease: a randomized, double-blind, placebo-controlled phase 3 trial. Nat Med. 2023;29(11):2919–28.
Younossi ZM, Golabi P, de Avila L, Paik JM, Srishord M, Fukui N, Qiu Y, Burns L, Afendy A, Nader F. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: a systematic review and meta-analysis. J Hepatol. 2019;71(4):793–801.
Lavine JE, Schwimmer JB, Van Natta ML, Molleston JP, Murray KF, Rosenthal P, Abrams SH, Scheimann AO, Sanyal AJ, Chalasani N, et al. Effect of vitamin E or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents: the TONIC randomized controlled trial. JAMA. 2011;305(16):1659–68.
Mantovani A, Byrne CD, Scorletti E, Mantzoros CS, Targher G. Efficacy and safety of anti-hyperglycaemic drugs in patients with non-alcoholic fatty liver disease with or without diabetes: an updated systematic review of randomized controlled trials. Diabetes Metab. 2020;46(6):427–41.
Musso G, Gambino R, Cassader M, Pagano G. A meta-analysis of randomized trials for the treatment of nonalcoholic fatty liver disease. Hepatology. 2010;52(1):79–104.
Sheka AC, Adeyi O, Thompson J, Hameed B, Crawford PA, Ikramuddin S. Nonalcoholic steatohepatitis: a review. JAMA. 2020;323(12):1175–83.
Ferguson D, Finck BN. Emerging therapeutic approaches for the treatment of NAFLD and type 2 diabetes mellitus. Nat Rev Endocrinol. 2021;17(8):484–95.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by National Natural Science Foundation Major International (Regional) Joint Research Program (82320108003), National Natural Science Foundation (82170845, 82000740, 81970717), the Key Research & Development Program (No.BE2022853) and Medical Key Discipline (ZDXK202203) of Jiangsu Province.
Author contributions
Conceptualization and methodology: Yifan Wang, He Yi, Weixia Sun, Hekai Yu, Wenxuan Tao and Ling Li; Data curation, formal analysis, visualization and validation: Yifan Wang, He Yi, Weixia Sun and Xiaojin Yu; Writing–original draft: Yifan Wang, He Yi; Writing–review & editing: Stephen J. Pandol, Dianrong Jia, Yingzhao Liu, Ling Li; Supervision: Weixia Sun, Dianrong Jia, Yingzhao Liu, Ling Li. All authors have accessed and verified the underlying data reported in the manuscript.
Conflict of interest
Yifan Wang has no potential conflicts of interest to declare for this article. He Yi has no potential conflicts of interest to declare for this article. Weixia Sun has no potential conflicts of interest to declare for this article. Hekai Yu has no potential conflicts of interest to declare for this article. Wenxuan Tao has no potential conflicts of interest to declare for this article. Xiaojin Yu has no potential conflicts of interest to declare for this article. Dianrong Jia has no potential conflicts of interest to declare for this article. Yingzhao Liu has no potential conflicts of interest to declare for this article. Stephen J. Pandol has no potential conflicts of interest to declare for this article. Ling Li has no potential conflicts of interest to declare for this article.
Data availability statements
Data are available upon reasonable request.
Code availability
Not applicable.
Ethics approval and patient consent for publication
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
40265_2024_2015_MOESM1_ESM.docx
Supplementary file1 Online Resource 1 This file provides information on effects of drugs on resolution of NASH with no worsening of liver fibrosis, the improvement of liver fibrosis by at least one stage with no worsening of NASH and a comprehensive description of effects of drugs on histology, imaging and serology (DOCX 32 KB)
40265_2024_2015_MOESM2_ESM.docx
Supplementary file2 Online Resource 2 This file depicts league tables of pairwise comparisons of drug therapies for NASH resolution with no worsening of fibrosis and the improvement of liver fibrosis stage (≥1) (DOCX 31 KB)
40265_2024_2015_MOESM3_ESM.docx
Supplementary file3 Online Resource 3 This file depicts risk of bias of RCTs included and provides funnel plots of studies included in the network meta-analyses and tests for inconsistency in network analysis (DOCX 1431 KB)
40265_2024_2015_MOESM4_ESM.docx
Supplementary file4 Online Resource 4 This file provides forest plots of estimates of pharmacological interventions on blood lipids, including low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides, and total cholesterol. This file also provides forest plots of estimates of changes in liver fibrosis stage and changes in liver fat content (DOCX 2468 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, Y., Yi, H., Sun, W. et al. Comparative Efficacy of Drug Interventions on NAFLD Over 24 Weeks: A Traditional and Network Meta-Analysis of Randomized Controlled Trials. Drugs (2024). https://doi.org/10.1007/s40265-024-02015-6
Accepted:
Published:
DOI: https://doi.org/10.1007/s40265-024-02015-6