Abstract
Background
HSK3486 (ciprofol), a new candidate drug similar to propofol, exerts sedative and hypnotic effects through gamma-aminobutyric acid type A receptors; however, its potential role in colorectal cancer is currently unknown.
Aims
This study aimed to evaluate the effects of HSK3486 on colorectal cancer cell proliferation.
Methods
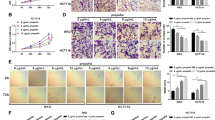
Imaging was performed to detect reactive oxygen species and mitochondrial membrane potential. Western blotting was used to determine the expression of target signals. The HSK3486 molecular mechanism was investigated through ATPase inhibitory factor 1 knockdown and xenograft model experiments to assess mitochondrial function in colorectal cancer cells.
Results
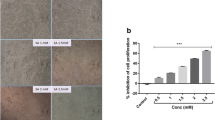
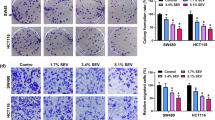
Cell Counting Kit-8 and Annexin V/propidium iodide double staining assays showed that HSK3486 inhibited colorectal cancer cell proliferation in a concentration-dependent manner. In addition, HSK3486 treatment increased the expression of B-cell lymphoma-2-associated X, cleaved caspase 3, and cleaved poly (ADP-ribose) polymerase, whereas myeloid cell leukemia-1 and B-cell lymphoma 2 expression decreased. HSK3486 promoted mitochondrial dysfunction by inducing ATPase inhibitor factor 1 expression. Furthermore, HSK3486 promoted oxidative stress, as shown by the increase in reactive oxygen species and lactate dehydrogenase levels, along with a decrease in mitochondrial membrane potential and ATP levels. ATPase inhibitor factor 1 small interfering RNA pretreatment dramatically increased the mitochondrial membrane potential and tumor size in a xenograft model following exposure to HSK3486.
Conclusion
Collectively, our findings revealed that HSK3486 induces oxidative stress, resulting in colorectal cancer cell apoptosis, making it a potential candidate therapeutic strategy for colorectal cancer.








Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in the published article.
References
Sung H, Ferlay J, Siegel RL et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209–249.
de Martel C, Georges D, Bray F, Ferlay J, Clifford GM. Global burden of cancer attributable to infections in 2018: a worldwide incidence analysis. Lancet Glob Health. 2020;8:e180–e190.
Bergers G, Fendt SM. The metabolism of cancer cells during metastasis. Nat Rev. Cancer. 2021;21:162–180.
Kroemer G, Pouyssegur J. Tumor cell metabolism: cancer’s Achilles’ heel. Cancer cell. 2008;13:472–482.
Bonora M, Patergnani S, Rimessi A et al. ATP synthesis and storage. Purinergic Signal. 2012;8:343–357.
García-Bermúdez J, Cuezva JM. The ATPase Inhibitory Factor 1 (IF1): a master regulator of energy metabolism and of cell survival. Biochim Biophys Acta. 2016;1857:1167–1182.
Tomasetig L, Di Pancrazio F, Harris DA, Mavelli I, Lippe G. Dimerization of F0F1ATP synthase from bovine heart is independent from the binding of the inhibitor protein IF1. Biochim Biophys Acta. 2002;1556:133–141.
Chen X, Wu Q, Sun P et al. Propofol disrupts aerobic glycolysis in colorectal cancer cells via inactivation of the NMDAR-CAMKII-ERK pathway. Cell Physiol Biochem. 2018;46:492–504.
Jacob Z, Li H, Makaryus R et al. Metabolomic profiling of children’s brains undergoing general anesthesia with sevoflurane and propofol. Anesthesiology. 2012;117:1062–1071.
Xu Y, Gao G, Sun X, Liu Q, Li C. ATPase inhibitory factor 1 is critical for regulating sevoflurane-induced microglial inflammatory responses and caspase-3 activation. Front Cell Neurosci. 2021;15:770666.
Ow YP, Green DR, Hao Z, Mak TW. Cytochrome c: functions beyond respiration. Nat Rev Mol Cell Biol. 2008;9:532–542.
Zhang S, Rao S, Yang M et al. Role of mitochondrial pathways in cell apoptosis during He-patic ischemia/reperfusion injury. Int J Mol Sci. 2022;23:2357.
Wang H, Zhao L, Wu J, Hong J, Wang S. Propofol induces ROS-mediated intrinsic apoptosis and migration in triple-negative breast cancer cells. Oncol Lett. 2020;20:810–816.
Tan SH, Ding HJ, Mei XP et al. Propofol suppressed cell proliferation and enhanced apoptosis of bladder cancer cells by regulating the miR-340/CDK2 signal axis. Acta Histochem. 2021;123:151728.
Su Z, Liu HL, Qi B, Liu Y. Effects of propofol on proliferation and apoptosis of cardia cancer cells via MAPK/ERK signaling pathway. Eur Rev Med Pharmacol Sci. 2020;24:428–433.
Ye LL, Cheng ZG, Cheng XE, Huang YL. Propofol regulates miR-1-3p/IGF1 axis to inhibit the proliferation and accelerates apoptosis of colorectal cancer cells. Toxicol Res. 2021;10:696–705.
Hu C, Ou X, Teng Y et al. Sedation effects produced by a ciprofol initial infusion or bolus dose followed by continuous maintenance infusion in healthy subjects: a phase 1 trial. Adv Ther. 2021;38:5484–5500.
Bhattacharya D, Gawali VS, Kallay L et al. Therapeutically leveraging GABA(A) receptors in cancer. Exp Biol Med. 2021;246:2128–2135.
Kleinerman RA, Brinton LA, Hoover R, Fraumeni JF Jr. Diazepam use and progression of breast cancer. Cancer Res. 1984;44:1223–1225.
Chen W, Di Z, Chen Z et al. NBPF4 mitigates progression in colorectal cancer through the regulation of EZH2-associated ETFA. J Cell Mol Med. 2021;25:9038–9050.
Maxwell DP, Wang Y, McIntosh L. The alternative oxidase lowers mitochondrial reactive oxygen production in plant cells. Proc Natl Acad Sci USA 1999;96:8271–8276.
Zhu H, Tan Y, Du W et al. Phosphoglycerate mutase 5 exacerbates cardiac ischemia-reperfusion injury through disrupting mitochondrial quality control. Redox Biol. 2021;38:101777.
Nan K, Han Y, Fang Q et al. HMGB1 gene silencing inhibits neuroinflammation via down-regulation of NF-κB signaling in primary hippocampal neurons induced by Aβ(25–35). Int Immunopharmacol. 2019;67:294–301.
Han Y, Chen R, Lin Q et al. Curcumin improves memory deficits by inhibiting HMGB1-RAGE/TLR4-NF-κB signalling pathway in APPswe/PS1dE9 transgenic mice hippocampus. J Cell Mol Med. 2021;25:8947–8956.
Trachootham D, Zhou Y, Zhang H et al. Selective killing of oncogenically transformed cells through a ROS-mediated mechanism by beta-phenylethyl isothiocyanate. Cancer Cell. 2006;10:241–252.
Esparza-Moltó PB, Nuevo-Tapioles C, Cuezva JM. Regulation of the H(+)-ATP synthase by IF1: a role in mitohormesis. Cell Mol Life Sci. 2017;74:2151–2166.
Xie YH, Chen YX, Fang JY. Comprehensive review of targeted therapy for colorectal cancer. Signal Transduct Target Ther. 2020;5:22.
Su Z, Hou XK, Wen QP. Propofol induces apoptosis of epithelial ovarian cancer cells by upregulation of microRNA let-7i expression. Eur J Gynaecol Oncol. 2014;35:688–691.
Zhang J, Wu GQ, Zhang Y, Feng ZY, Zhu SM. Propofol induces apoptosis of hepatocellular carcinoma cells by upregulation of microRNA-199a. Cell Biol Int. 2013;37:227–232.
Yu B, Gao W, Zhou H et al. Propofol induces apoptosis of breast cancer cells by downregulation of miR-24 signal pathway. Cancer Biomark. 2018;21:513–519.
Desagher S, Martinou JC. Mitochondria as the central control point of apoptosis. Trends Cell Biol. 2000;10:369–377.
Kang FC, Chen YC, Wang SC, So EC, Huang BM. Propofol induces apoptosis by activating caspases and the MAPK pathways, and inhibiting the Akt pathway in TM3 mouse Leydig stem/progenitor cells. Int J Mol Med. 2020;46:439–448.
Kopeina GS, Prokhorova EA, Lavrik IN, Zhivotovsky B. Alterations in the nucleocytoplasmic transport in apoptosis: caspases lead the way. Cell Prolif. 2018;51:e12467.
Chen BZ, Yin XY, Jiang LH et al. The efficacy and safety of ciprofol use for the induction of general anesthesia in patients undergoing gynecological surgery: a prospective randomized controlled study. BMC Anesthesiol. 2022;22:245.
Liu Y, Yu X, Zhu D et al. Safety and efficacy of ciprofol vs. propofol for sedation in intensive care unit patients with mechanical ventilation: a multi-center, open label, randomized, phase 2 trial. Chin Med J. 2022;135:1043–1051.
Zhu Q, Luo Z, Wang X et al. Efficacy and safety of ciprofol versus propofol for the induction of anesthesia in adult patients: a multicenter phase 2a clinical trial. Int J Clin Pharm. 2023;45:473–482.
Liao J, Li M, Huang C et al. Pharmacodynamics and pharmacokinetics of HSK3486, a novel 2,6-disubstituted phenol derivative as a general anesthetic. Front Pharmacol. 2022;13:830791.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674.
Muravchick S, Levy RJ. Clinical implications of mitochondrial dysfunction. Anesthesiology. 2006;105:819–837.
Seo EH, Piao L, Park HJ et al. Impact of general anaesthesia on endoplasmic reticulum stress: propofol vs. isoflurane. Int J Med Sci. 2019;16:1287–1294.
Lisowska B, Szymańska M, Nowacka E, Olszewska M. Anesthesiology and the cytokine network. Postępy Hig Med Dośw. 2013;67:761–769.
Qin J, Li Y, Wang K. Propofol induces impairment of mitochondrial biogenesis through inhibiting the expression of peroxisome proliferator-activated receptor-γ coactivator-1α. J Cell Biochem. 2019;120:18288–18297.
Zou J, Zhang Y, Sun J et al. Deoxyelephantopin induces reactive oxygen species-mediated apoptosis and autophagy in human osteosarcoma cells. Cell Physiol Biochem. 2017;42:1812–1821.
Gallo M, Sapio L, Spina A et al. Lactic dehydrogenase and cancer: an overview. Front Biosci. 2015;20:1234–1249.
Franke RP, Fuhrmann R, Mrowietz C et al. Reduced diagnostic value of lactate dehydrogenase (LDH) in the presence of radiographic contrast media. Clin Hemorheol Microcirc. 2010;45:123–130.
Chen WW, Birsoy K, Mihaylova MM et al. Inhibition of ATPIF1 ameliorates severe mitochondrial respiratory chain dysfunction in mammalian cells. Cell Rep. 2014;7:27–34.
Prieto J, Seo AY, León M et al. MYC induces a hybrid energetics program early in cell reprogramming. Stem Cell Rep. 2018;11:1479–1492.
Matsuyama S, Xu Q, Velours J, Reed JC. The Mitochondrial F0F1-ATPase proton pump is required for function of the proapoptotic protein Bax in yeast and mammalian cells. Mol Cell. 1998;1:327–336.
Bonora M, Wieckowski MR, Chinopoulos C et al. Molecular mechanisms of cell death: central implication of ATP synthase in mitochondrial permeability transition. Oncogene. 2015;34:1475–1486.
Kwong JQ, Molkentin JD. Physiological and pathological roles of the mitochondrial permeability transition pore in the heart. Cell Metab. 2015;21:206–214.
Wei S, Fukuhara H, Kawada C et al. Silencing of ATPase inhibitory factor 1 inhibits cell growth via cell cycle arrest in bladder cancer. Pathobiology. 2015;82:224–232.
Zhang C, Min L, Liu J et al. Integrated analysis identified an intestinal-like and a diffuse-like gene sets that predict gastric cancer outcome. Tumour Biol. 2016;37:16317–16335.
Giorgio V, Burchell V, Schiavone M et al. Ca(2+) binding to F-ATP synthase β subunit triggers the mitochondrial permeability transition. EMBO Rep. 2017;18:1065–1076.
Chidambaran V, Costandi A, D’Mello A. Propofol: a review of its role in pediatric anesthesia and sedation. CNS Drugs. 2015;29:543–563.
Sahinovic MM, Struys M, Absalom AR. Clinical pharmacokinetics and pharmacodynamics of propofol. Clin Pharmacokinet. 2018;57:1539–1558.
Teng Y, Ou M, Wang X et al. Efficacy and safety of ciprofol for the sedation/anesthesia in patients undergoing colonoscopy: phase IIa and IIb multi-center clinical trials. Eur J Pharm Sci. 2021;164:105904.
Acknowledgments
This work was financially supported by the National Key Research and Development Program of China (No. 2020YFC2008400), the National Natural Science Foundation of China (No. 82072213, 81873948), the Clinical Research Plan of SHDC (No. SHDC2020CR1005A, SHDC2020CR4064), Shanghai Leading Talent (No. 2019-112), the Shanghai Municipal 2021 Science and Technology Innovation Action Plan (No. 21JC1401400). The authors would like to acknowledge the support of the Intelligent Medical Service Project of Zhongshan Hospital (No. 2020ZHZS25, Shanghai, China).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analyses were performed by KN, ZZ and YY. The first draft of the manuscript was written by ZZ and KN, and JZ, CM and MS conceived and supervised the revision of the manuscript. All authors commented on the previous versions of the manuscript and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no financial or proprietary interests in any of the materials discussed in this article.
Ethical approval
All experiments were approved by the Animal Ethics Committee of the Institute Ethics Committee of Zhongshan Hospital, Fudan University, and the Use of Laboratory Animals (license: 202011009S).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nan, K., Zhong, Z., Yue, Y. et al. HSK3486 Inhibits Colorectal Cancer Growth by Promoting Oxidative Stress and ATPase Inhibitory Factor 1 Activation. Dig Dis Sci 69, 1214–1227 (2024). https://doi.org/10.1007/s10620-023-08213-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-023-08213-8