Abstract
Background
An increasing number of women of reproductive age are treated with attention-deficit hyperactivity disorder (ADHD) medication; however, patterns of ADHD medication use for women in the perinatal period have not been well described.
Objective
This study aimed to describe ADHD medication use patterns from 1 year before pregnancy to 1 year after delivery, and to describe sociodemographic characteristics and clinical features by medication trajectories.
Methods
The population-based cohort study included pregnancies in Denmark between 1997 and 2020, from the Medical Birth Register, by women who filled at least one prescription for ADHD medication from 12 months before pregnancy until 12 months after delivery. We applied group-based trajectory modeling to classify women into subgroups based on the identification of heterogeneous ADHD medication treatment patterns, and described the characteristics associated with these groups.
Results
Overall, we included 4717 pregnancies leading to liveborn singletons by 4052 mothers with a mean (standard deviation) age of 27.5 (5.6) years. We identified four treatment trajectories across pregnancy and the postpartum period: continuers (23.3%), discontinuers (41.8%), interrupters who ceased filling prescriptions during pregnancy but resumed postpartum (17.2%), and postpartum initiators (17.7%). Continuers were older at the time of conception, gave birth in more recent years, were more likely to smoke during pregnancy, and used other psychotropic medications during pregnancy. A large proportion of continuers used methylphenidate (89.1%) compared with the other groups (75.9–84.1%) and had switched ADHD medication type during the whole period (16.4% vs. 7.4–14.8%).
Conclusion
We found that approximately 60% of women discontinued or interrupted their ADHD medication around pregnancy, and those who continued differed in sociodemographic and clinical factors that may reflect more severe ADHD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
This population-based cohort study of attention-deficit hyperactivity disorder (ADHD) medication use among women in the perinatal period showed four distinct trajectories: continuers (23.3%), discontinuers (41.8%), interrupters (17.2%), and postpartum initiators (17.7%). |
More than half of all women discontinued ADHD medication during pregnancy. |
The sociodemographic and clinical characteristics of the continuers and the initiators may reflect more severe ADHD. |
1 Introduction
Attention-deficit hyperactivity disorder (ADHD) is a neurodevelopmental disorder characterized by symptoms of hyperactivity-impulsivity and/or inattention that causes considerable impairment in daily life [1]. ADHD affects approximately 5% of children and 4% of adults [2, 3], and up to 60% of children with the disorder continue to experience clinically significant symptoms in adulthood [4]. Until recently, ADHD in girls has often been overlooked, and the diagnoses are delayed in females [5, 6]. However, with increasing recognition of ADHD as a common and impairing disorder in females [6], more girls and women worldwide are now being diagnosed and treated for the disorder [7].
Symptoms of ADHD can significantly impact an individual’s daily functioning, including work performance, social relationships, reproductive behaviors, and parenting skills [8,9,10]. Stimulant and non-stimulant medications are commonly used in the treatment of ADHD and, particularly stimulants, have been shown to be effective in reducing symptoms in both children and adults [11]. Additionally, stimulant medication treatment has been associated with reduced risk of mood disorders, suicidality, criminality, substance use disorders, accidents, injuries, and poor educational outcomes in people with ADHD [12]. Over the past decade, an increasing number of women of reproductive age have received ADHD medication treatment [13]. In the United States, the prevalence of ADHD medication use among women aged 15–44 years increased from 0.9% in 2003 to 4% in 2015 [7], with similar patterns observed in the Nordic countries [14]. Although the prevalence of ADHD medication use during pregnancy is still relatively low, there is an increasing trend in the Nordic countries; in Sweden, the prevalence increased from 1.6/1000 in 2010 to 7.9/1000 in 2019, and from 2.5 to 5.5 in Norway [15]. Worryingly, there are no established guidelines for administering ADHD medication during the perinatal period, and there is a scarcity of empirical evidence regarding its effects on pregnancy and fetal outcomes [8, 16]. Moreover, there is limited knowledge about medication treatment patterns of women with ADHD, specifically during pregnancy and the postpartum period. When studying health outcomes after medication exposure during pregnancy, individuals are typically classified as either exposed or non-exposed; however, health outcomes may vary based on the specific pattern of medication use, highlighting a critical knowledge gap. It is essential to address this gap, as medication treatment decisions during these periods can have significant implications for both maternal and fetal/neonatal health. A recent study from Sweden and Norway found that ADHD medication use among women around pregnancy and postpartum follows multiple distinct patterns, with the majority discontinuing or pausing ADHD medication use around pregnancy, and continuation being rare [17]. These results need to be replicated to provide external validation. It is of interest to assess whether the patterns of use and their prevalences are similar in Denmark, as the prevalence of medication treatment patterns with antidepressants before and during the perinatal period have been reported to differ between the Nordic countries [18].
In this study, we aimed to describe medication treatment patterns among women with ADHD before pregnancy, during pregnancy, and after delivery by applying group-based trajectories to nationwide register data from Denmark. Furthermore, we examined demographic and clinical factors potentially associated with ADHD medication treatment patterns.
2 Methods
2.1 Study Population
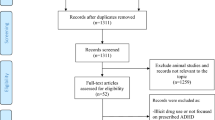
We conducted a population-based cohort study utilizing Danish nationwide registers. All residents in Denmark are assigned a unique personal identification number, enabling data linkage between registers. We first identified all pregnancies leading to singleton live births between 1997 and 2020 from the Danish Medical Birth Register (N = 1,472,383) [19]. To ensure data accuracy, we excluded 28,093 (1.9%) pregnancies with missing or extreme gestational age (<154 days or >315 days), as well as 3897 (0.3%) pregnancies by women who emigrated or died within 12 months after delivery to ensure data completeness for ADHD medication use in the defined period (Fig. 1).
2.2 Attention-Deficit Hyperactivity Disorder (ADHD) Medication
We obtained data on ADHD medication dispensations from the Danish National Prescription Registry, which provides information on the Anatomical Therapeutic Chemical (ATC) classification codes and the date of prescriptions filled in community pharmacies in Denmark since 1995 [20]. We included the following ATC codes to identify all medications that are prescribed for the treatment of ADHD in adults [11] in line with previous work [21] acknowledging that some are third- or fourth-line treatments for ADHD: stimulant ATC codes N06BA01, -02, -04, and -12 (amfetamine, dexamfetamine, methylphenidate, and lisdexamfetamine, respectively), and non-stimulant ATC codes N06BA07 and -09, C02AC01 and -02 (modafinil, atomoxetine, clonidine, and guanfacine, respectively).
To define ADHD medication treatment status, we identified women who had filled a prescription within 3-month intervals spanning from 12 months prior to time of conception to 12 months after delivery, totaling 33 months and comprising 11 estimation periods (including the 9 months of pregnancy). The 11 estimation periods are (1) from 12 to 9 months before pregnancy start; (2) from 9 to 6 months before; (3) from 6 to 3 months before; (4) from 3 months to pregnancy start; (5) first trimester; (6) second trimester; (7) third trimester; (8) from delivery to 3 months postpartum: (9) 3–6 months postpartum; (10) 6–9 months postpartum; and (11) 9–12 months postpartum. Exposure to ADHD medication was coded as a binary variable (1) if the dispensation date occurred within the 3-month interval, or 0 otherwise. Date of conception was calculated by subtracting gestational age from the date of birth obtained from the Danish Medical Birth Register. Gestational age at birth was derived from ultrasound scans during the first or second trimester, or, if missing, conception was established using the initial day of the mother's last menstrual period [19].
2.3 Sociodemographic and Health-Related Maternal Characteristics
We analyzed clinically relevant sociodemographic and health-related characteristics of the mothers to explore their association with ADHD medication use trajectory during pregnancy and the postpartum period. Maternal age at the start of pregnancy (<25, 25–34, ≥35 years), primiparity (yes/no), smoking during pregnancy (yes/no), and calendar year of pregnancy (1996–2005, 2006–2010, 2011–2015, or 2016–2020) were retrieved from the Danish Medical Birth Registry. Marital status (married or co-habiting, single, divorced or widowed), and level of education (mandatory school, above mandatory school) in the year of pregnancy were retrieved from Statistics Denmark's socioeconomic registers [22]. We also considered clinical characteristics, including age at the first ADHD diagnosis, previous prescriptions of stimulants (ATC codes N06BA01, -02, -04, -12) and non-stimulants (ATC codes N06BA07 and -09, C02AC01 and -02), and other diagnosed psychiatric disorders (substance abuse disorders, psychotic disorders, mood/affective disorders, anxiety and stress-related disorders, eating disorders, sleep disorders, personality disorders, and other psychiatric disorders) any time prior to the trajectory period (12 months before pregnancy), inpatient and outpatient treatment for psychiatric disorders and somatic disorders in the year preceding the trajectory period, and sex of the child. ADHD onset was defined as the first ADHD diagnosis recorded in the Psychiatric Central Research Register or the first date of filling an ADHD medication, whichever came first. We also examined co-prescribed psychotropic medications (antidepressants, antipsychotics, opioid analgesics, anxiolytics and hypnotics, antiseizure medications, and drugs used in alcohol or opioid dependence), including any date for redeemed prescriptions from 12 months prior to pregnancy to 12 months after delivery. We considered a woman exposed to a co-prescribed medication if the date of dispensation occurred within a specific 3-month interval. Additional information on the International Classification of Diseases (ICD) Eighth Revision (ICD-8) and Tenth Revision (ICD-10) codes (ICD Ninth Revision [ICD-9] was never implemented in Denmark) for psychiatric diagnoses, and ATC codes for co-prescribed medications, can be found in Online Resource Table 1 and Table 2, respectively.
2.4 Statistical Analysis
We applied group-based trajectory modeling to classify women into subgroups based on the identification of heterogeneous ADHD medication treatment patterns, using ‘traj’ in Stata version 16.0 (StataCorp LLC, College Station, TX, USA). We fitted group-based trajectory models with one to six groups and tested each model with linear, quadratic, and cubic terms to determine the best shapes that fit the data. We then decided the optimal number of trajectory groups based on four criteria: (1) Bayesian information criterion (BIC) and Akaike Information Criteria [23], with a lower BIC and AIC indicating a better model fit [24]; the BIC log Bayes factor approximation was defined as 2 × [ΔBIC] (subtracting a less complex model from a more complex model), and 2 × [ΔBIC] higher than 10 is considered solid evidence in favor of the more complex model; (2) an average posterior probability of ≥0.7 in each group identified; (3) a trajectory group had to constitute ≥10% of the total sample; and (4) clinical relevance, i.e., whether the trajectory groups have practical utility in guiding treatment decisions or interventions. Women were assigned to the trajectory with the maximum posterior group probability [25].
2.5 Sensitivity Analyses
In a sensitivity analysis, we restricted the analyses to 2710 primiparous pregnancies to address the concern about any dependency between pregnancies by the same woman. Furthermore, we restricted our analysis to 4344 term-born children to evaluate the potential impact of gestational age on the trajectories.
In reporting the study, we followed the Strengthening the Reporting of Observational studies in Epidemiology (STROBE) guidelines.
3 Results
We included 4717 (0.33%) singletons born to 4052 women who filled at least one ADHD medication prescription from 12 months before pregnancy until 12 months after delivery during 1997–2020. The mean age (standard deviation) at the first day of pregnancy was 27.5 (5.6) years, 57.5% were primiparous, and 34.2% were diagnosed with ADHD before age 20 years.
3.1 ADHD Treatment Trajectory Description
A model with four trajectories provided the best model fit according to the selection criteria outlined in Online Resource Table 3. The average posterior probabilities for all four groups were within an acceptable range of 0.77–0.92. The four groups were identified and named based on their initial position and subsequent trajectory, as depicted in Fig. 2: (1) continuers (23.3%): this subgroup filled at least one ADHD medication prescription in every 3-month interval before, during pregnancy, and in the first year postpartum; (2) discontinuers (41.8%): this subgroup filled ADHD prescriptions in the 12 months before pregnancy and stopped filling ADHD medication prescriptions during pregnancy; (3) interrupters (17.2%): this subgroup reduced filling prescriptions during pregnancy but resumed postpartum; and (4) initiators (17.7%): this subgroup did not fill ADHD medication prescriptions before pregnancy but began postpartum.
3.2 Characteristics and Clinical Features According to ADHD Medication Treatment Trajectories
Table 1 presents an overview of the characteristics and clinical features based on fill trajectories. The continuers tended to be older at the start of pregnancy (15.8% were aged 35 years or older vs. 11.4–12.2% in the other groups), more likely to smoke during pregnancy (40.1% vs. 35.2–38.5% in the other groups), and had their index pregnancy in more recent years (52.8% from 2016 to 2020 vs. 35.8–46.1% in the other groups). A larger proportion of continuers used methylphenidate (89.1%) compared with the other groups (75.9–84.1%) and had switched ADHD medication type during the whole period (16.4% vs. 7.4–14.8%). Continuers were diagnosed with ADHD at a later age (36.8% at age 25 years or older) compared with discontinuers (32.3%) and interrupters (31.9%), and more had prior prescriptions for both stimulant (72.8% vs. 25.5–63.9% in the other groups) and non-stimulant medications (28.6% vs. 10.5–27.3% in the other groups). All groups largely followed the same pattern in terms of filling other psychotropic medications with a decrease around pregnancy, but the continuers had, to a larger extent, filled other psychotropic medications overall in the period, while the discontinuers had the lowest level from pregnancy and onwards (Fig. 3). For initiators, more than half had previous pregnancies (55.9%), 53.6% were aged 25 years or above when first diagnosed with ADHD, 45.1% had redeemed prescriptions for antidepressants in the period before, during and after pregnancy, while only a few had previous prescriptions of stimulants (25.5%) and non-stimulants (10.5%) prior to 12 months before pregnancy. Finally, initiators had the lowest frequency of women who had inpatient or outpatient psychiatric contacts (2.8% and 13%, respectively) the year preceding the trajectory period, while they had the highest frequency of inpatient or outpatient contacts for non-psychiatric disorders (31.8% and 63.2%, respectively).
3.3 Sensitivity Analyses
When restricting the analyses to 2710 primiparous pregnancies, we consistently observed the same four ADHD medication-filling trajectories (Online Resource Fig. 1). However, the distribution in trajectories varied from the primary analyses with fewer continuers or initiators and more discontinuers (continuers [16.8%], discontinuers [48.1%], interrupters [21.5%], and initiators [13.6%]). To evaluate the potential impact of gestational age on the trajectory patterns, we examined exclusively term-born children (N = 4344). Here, we also observed four medication-filling trajectories (Online Resource Fig. 2), although with fewer continuers and more interrupters (continuers [18.1], discontinuers [42.3%], interrupters [22.1%], and initiators [17.5%]).
4 Discussion
In the present study, we included 4717 pregnancies among 4052 women who filled at least one ADHD medication prescription from 12 months before pregnancy until 12 months after delivery during the period 1997–2020. We identified four ADHD medication treatment trajectories: continuers (23.3%), discontinuers (41.8%), interrupters (17.2%), and initiators (17.7%).
While almost 60% of the women discontinued ADHD medication use, only 17% of them re-initiated (interrupters) after giving birth. Although the discontinuation group had a similar pattern of using other psychotropic medication the year before pregnancy as the other groups, they discontinued other psychotropic medication more frequently. Discontinuing psychotropic medication during pregnancy and throughout the lactation period may have been influenced by a motivation to minimize fetal exposure as well as infant exposure through breastfeeding. However, not re-initiating ADHD medication could also reflect a fragile group of women with limited access to psychiatric care.
The distinct adverse profile of the continuers may reflect a more vulnerable and more severely impaired group who might be more difficult to treat, with more shifts in medication and use of other psychotropic drugs. Hence, they might be more strongly advised to stay on medication and also more rigorously followed up by their psychiatrist not only due to their ADHD but also due to other psychiatric conditions. However, this group could also consist of women with very few adverse effects, better treatment response, and taking low doses of medication for longer periods that would make them more likely to stay in treatment. Refilling prescriptions and maintaining contact with the physician is required for consistent treatment, which is not normally consistent with a ‘more severe’ ADHD.
Surprisingly, the group of initiators constituted almost 18% of the women. In this group, women redeemed ADHD medication in late pregnancy or after delivery and only a few had ever previously filled prescriptions for ADHD medication. There may be different possible reasons for this, and one could be an indication that the challenges that come with pregnancy and motherhood might have increased the need for treatment. Pregnancy and motherhood can be a straining period associated with emotional, physical and social change, and there is substantial evidence that childbirth increases the risk of mental disorders in women in general, particularly in women with pre-existing psychiatric conditions [26]. In this group, the majority were diagnosed with ADHD late (77% after age 20 years), but almost half had filled antidepressants in the period before, during, or after pregnancy. Furthermore, while only few had had psychiatric hospital contact in the year preceding the trajectory period, the proportion of women having had inpatient or outpatient hospital contacts for non-psychiatric disorders was far larger than what was seen in the other groups. Together, this may indicate a group of women who were affected by both somatic and psychiatric comorbid disorders potentially as a result of a delayed ADHD diagnosis [6].
We identified four similar trajectory patterns as a recent study of ADHD medication trajectories using Norwegian and Swedish register data [17]; however, the proportion of pregnancies classified in each of the trajectory patterns varied, with a notably higher proportion of continuers in Denmark. This may be partly driven by the specification of the models, which resulted in a more highly selected group defined as continuers in Norway and Sweden (5.7%), with a higher proportion of women using ADHD medication in each period (around 80–90%). However, the characteristics of the continuers were similar in both studies. Those who continued in Norway and Sweden were also older, more likely to smoke during pregnancy, and had higher use of other classes of psychotropic medication compared with other groups. However, in contrast to our findings, methylphenidate was the most prescribed ADHD medication overall, while continuers were least likely to use it in Norway and Sweden, likely driven by the increasing use of lisdexamfetamine in these countries [15]. The classification of ADHD medication also differs between the studies as we have included other non-stimulants. We observed that continuers were most likely to switch medication in the study period relative to other groups, suggesting a more dynamic treatment approach in Denmark. Moreover, more women in the continuation group used lisdexamfetamine, which may be related to them being diagnosed in recent years, where an overall increase in use of this medication type has been observed [15]. Cross-country differences in the proportion of continuers may also in part be attributable to differences in prescriber attitudes and specialty care access. This pattern is also observed for antidepressant treatment during pregnancy, where the prevalence of continuers is larger in Denmark than in Norway [18].
In the last decade, a notable increase in the utilization of ADHD medication has been observed, spanning over both the general population and women of reproductive age [7, 14], including during pregnancy [15]. We observed the same trend in our study, with an increasing number of women who fill a prescription for ADHD medication before, during, or after pregnancy from 2011 onwards. Furthermore, the distribution in the trajectories has shifted towards more continuers in recent years. Although there are no clinical guidelines regarding ADHD medication use during pregnancy, the increase in prescription fills during pregnancy may reflect psychiatrists increasing willingness to prescribe ADHD medication to pregnant women, possibly due to the emergent evidence suggesting no risk or low absolute risk of adverse pregnancy, birth and offspring outcomes [27,28,29]. However, the lack of clinical guidelines will nevertheless result in an imbalanced treatment and guidance for women with ADHD dependent on the level of knowledge of each treating physician. Given the increasing number of females with ADHD entering reproductive age, there is an urgent need for international consensus to guide clinical decisions.
The main strength of this study is the use of nationwide register-based data, limiting selection bias to a high degree. Our study does have certain limitations worth noting. First, we focused on modeling filling trajectories. As a result, our findings may not entirely accurately reflect how the medication was used, and the trajectories are not directly observed but derived under certain modeling assumptions. Second, there is a risk of misclassification when using redeemed prescriptions to define exposure status, and underestimating medication use, when using prescription fills and not self-report for drugs that can be used episodically, has been reported [30]. Third, we included both modafinil and clonidine because these medications are used off-label in the treatment of ADHD when there is a lack of effect of the registered preparations despite that the medications do not have a registered indication for use in ADHD.
5 Conclusion
Around 60% of women using ADHD medication in the perinatal period discontinued or interrupted their treatment around pregnancy. Those who continued ADHD medication use differed in sociodemographic and clinical factors, in terms of greater use of other psychotropic medications, more smoking during pregnancy, and more switching ADHD medication type, which may reflect more severe ADHD. Finally, a large group of women initiated ADHD medication in late pregnancy or after delivery. This group was characterized by the majority being diagnosed late, a larger proportion in antidepressant treatment, and having had inpatient or outpatient hospital contacts for non-psychiatric disorders than any of the other groups.
References
Posner J, Polanczyk GV, Sonuga-Barke E. Attention-deficit hyperactivity disorder. The Lancet. 2020;395(10222):450–62. https://doi.org/10.1016/S0140-6736(19)33004-1.
Polanczyk G, de Lima MS, Horta BL, Biederman J, Rohde LA. The worldwide prevalence of ADHD: a systematic review and metaregression analysis. Am J Psychiatry. 2007;164(6):942–8. https://doi.org/10.1176/ajp.2007.164.6.942.
Kessler RC, Adler L, Barkley R, Biederman J, Conners CK, Demler O, et al. The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. Am J Psychiatry. 2006;163(4):716–23. https://doi.org/10.1176/ajp.2006.163.4.716.
Sibley MH, Swanson JM, Arnold LE, Hechtman LT, Owens EB, Stehli A, et al. Defining ADHD symptom persistence in adulthood: optimizing sensitivity and specificity. J Child Psychol Psychiatry. 2017;58(6):655–62. https://doi.org/10.1111/jcpp.12620.
Madsen KB, Ravn MH, Arnfred J, Olsen J, Rask CU, Obel C. Characteristics of undiagnosed children with parent-reported ADHD behaviour. Eur Child Adolesc Psychiatry. 2018;27(2):149–58. https://doi.org/10.1007/s00787-017-1029-4.
Young S, Adamo N, Ásgeirsdóttir BB, Branney P, Beckett M, Colley W, et al. Females with ADHD: an expert consensus statement taking a lifespan approach providing guidance for the identification and treatment of attention-deficit/ hyperactivity disorder in girls and women. BMC Psychiatry. 2020;20(1):404. https://doi.org/10.1186/s12888-020-02707-9.
Anderson KN, Ailes EC, Danielson M, Lind JN, Farr SL, Broussard CS, et al. Attention-deficit/hyperactivity disorder medication prescription claims among privately insured women aged 15–44 years—United States, 2003–2015. MMWR Morb Mortal Wkly Rep. 2018;67(2):66–70. https://doi.org/10.15585/mmwr.mm6702a3.
Kittel-Schneider S, Quednow BB, Leutritz AL, McNeill RV, Reif A. Parental ADHD in pregnancy and the postpartum period: a systematic review. Neurosci Biobehav Rev. 2021;124:63–77. https://doi.org/10.1016/j.neubiorev.2021.01.002.
Østergaard SD, Dalsgaard S, Faraone SV, Munk-Olsen T, Laursen TM. Teenage parenthood and birth rates for individuals with and without attention-deficit/hyperactivity disorder: a nationwide cohort study. J Am Acad Child Adolesc Psychiatry. 2017;56(7):578–84. https://doi.org/10.1016/j.jaac.2017.05.003. (e3).
Skoglund C, Kopp Kallner H, Skalkidou A, Wikström A-K, Lundin C, Hesselman S, et al. Association of attention-deficit/hyperactivity disorder with teenage birth among women and girls in Sweden. JAMA Netw Open. 2019;2(10): e1912463-e. https://doi.org/10.1001/jamanetworkopen.2019.12463.
Cortese S, Adamo N, Del Giovane C, Mohr-Jensen C, Hayes AJ, Carucci S, et al. Comparative efficacy and tolerability of medications for attention-deficit hyperactivity disorder in children, adolescents, and adults: a systematic review and network meta-analysis. Lancet Psychiatry. 2018;5(9):727–38. https://doi.org/10.1016/s2215-0366(18)30269-4.
Boland H, DiSalvo M, Fried R, Woodworth KY, Wilens T, Faraone SV, et al. A literature review and meta-analysis on the effects of ADHD medications on functional outcomes. J Psychiatr Res. 2020;123:21–30. https://doi.org/10.1016/j.jpsychires.2020.01.006.
Baker AS, Freeman MP. Management of attention deficit hyperactivity disorder during pregnancy. Obstet Gynecol Clin North Am. 2018;45(3):495–509. https://doi.org/10.1016/j.ogc.2018.04.010.
Karlstad Ø, Zoëga H, Furu K, Bahmanyar S, Martikainen JE, Kieler H, et al. Use of drugs for ADHD among adults-a multinational study among 15.8 million adults in the Nordic countries. Eur J Clin Pharmacol. 2016;72(12):1507–14. https://doi.org/10.1007/s00228-016-2125-y.
Cohen JM, Srinivas C, Furu K, Cesta CE, Reutfors J, Karlstad Ø. Prevalence trends and individual patterns of ADHD medication use in pregnancy in Norway and Sweden, 2010–2019. Eur J Clin Pharmacol. 2023;79(1):173–80. https://doi.org/10.1007/s00228-022-03428-6.
McAllister-Williams RH, Baldwin DS, Cantwell R, Easter A, Gilvarry E, Glover V, et al. British Association for Psychopharmacology consensus guidance on the use of psychotropic medication preconception, in pregnancy and postpartum 2017. J Psychopharmacol. 2017;31(5):519–52. https://doi.org/10.1177/0269881117699361.
Srinivas C, Karlstad Ø, Stigum H, Furu K, Cesta CE, Reutfors J, et al. Trajectories of ADHD medication use before, during, and after pregnancy: a population-based study from Norway and Sweden. Pharmacoepidemiol Drug Saf. 2023;32(10):1152–60. https://doi.org/10.1002/pds.5654.
Trinh NTH, Munk-Olsen T, Wray NR, Bergink V, Nordeng HME, Lupattelli A, et al. Timing of antidepressant discontinuation during pregnancy and postpartum psychiatric outcomes in Denmark and Norway. JAMA Psychiat. 2023;80(5):441–50. https://doi.org/10.1001/jamapsychiatry.2023.0041.
Bliddal M, Broe A, Pottegård A, Olsen J, Langhoff-Roos J. The Danish medical birth register. Eur J Epidemiol. 2018;33(1):27–36. https://doi.org/10.1007/s10654-018-0356-1.
Pottegård A, Schmidt SAJ, Wallach-Kildemoes H, Sørensen HT, Hallas J, Schmidt M. Data resource profile: the Danish national prescription registry. Int J Epidemiol. 2016;46(3):798-f. https://doi.org/10.1093/ije/dyw213.
Bang Madsen K, Robakis TK, Liu X, Momen N, Larsson H, Dreier JW, et al. In utero exposure to ADHD medication and long-term offspring outcomes. Mol Psychiatry. 2023;28(4):1739–46. https://doi.org/10.1038/s41380-023-01992-6.
Petersson F, Baadsgaard M, Thygesen LC. Danish registers on personal labour market affiliation. Scand J Public Health. 2011;39(7 Suppl):95–8. https://doi.org/10.1177/1403494811408483.
Kronish IM, Rieckmann N, Halm EA, Shimbo D, Vorchheimer D, Haas DC, et al. Persistent depression affects adherence to secondary prevention behaviors after acute coronary syndromes. J Gen Intern Med. 2006;21(11):1178–83. https://doi.org/10.1111/j.1525-1497.2006.00586.x.
Jones BL, Nagin DS, Roeder K. A SAS procedure based on mixture models for estimating developmental trajectories. Sociol Methods Res. 2001;29(3):374–93. https://doi.org/10.1177/0049124101029003005.
Herle M, Micali N, Abdulkadir M, Loos R, Bryant-Waugh R, Hübel C, et al. Identifying typical trajectories in longitudinal data: modelling strategies and interpretations. Eur J Epidemiol. 2020;35(3):205–22. https://doi.org/10.1007/s10654-020-00615-6.
Meltzer-Brody S, Howard LM, Bergink V, Vigod S, Jones I, Munk-Olsen T, et al. Postpartum psychiatric disorders. Nat Rev Dis Primers. 2018;4:18022. https://doi.org/10.1038/nrdp.2018.22.
Jiang H-Y, Zhang X, Jiang C-M, Fu H-B. Maternal and neonatal outcomes after exposure to ADHD medication during pregnancy: a systematic review and meta-analysis. Pharmacoepidemiol Drug Saf. 2019;28(3):288–95. https://doi.org/10.1002/pds.4716.
Huybrechts KF, Bröms G, Christensen LB, Einarsdóttir K, Engeland A, Furu K, et al. Association between methylphenidate and amphetamine use in pregnancy and risk of congenital malformations: a cohort study from the international pregnancy safety study consortium. JAMA Psychiat. 2018;75(2):167–75. https://doi.org/10.1001/jamapsychiatry.2017.3644.
Li L, Sujan AC, Butwicka A, Chang Z, Cortese S, Quinn P, et al. Associations of prescribed ADHD medication in pregnancy with pregnancy-related and offspring outcomes: a systematic review. CNS Drugs. 2020;34(7):731–47. https://doi.org/10.1007/s40263-020-00728-2.
Harris GE, Wood M, Nordeng H. Modeling exposures of medications used episodically during pregnancy: triptans as a motivating example. Pharmacoepidemiol Drug Saf. 2020;29(9):1111–9. https://doi.org/10.1002/pds.5089.
Funding
Open access funding provided by Aarhus Universitet.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by the National Institute of Mental Health (NIMH; R01MH122869). Partial financial support was also received from Sygeforsikring ‘danmark’ (Journalnr. 2021-0139). The funding agencies had no role in the design, data collection, analysis, interpretation, writing the manuscript, or the decision to submit the manuscript for publication.
Conflicts of Interest
Kathrine Bang Madsen has received a speaker’s fee from Medice Nordic within the last 3 years. Charlotte Borg Skoglund has served on advisory boards, as a speaker, and received honoraria from Shire/Takeda, Nordic Drugs, UCB Pharma, DNE Pharma, Novartis, Evolan, Lundbeck A/S and Medice. Henrik Larsson reports receiving grants from Shire Pharmaceuticals; personal fees from and serving as a speaker for Medice, Shire/Takeda Pharmaceuticals and Evolan Pharma AB; and sponsorship for a conference on ADHD from Shire/Takeda Pharmaceuticals and Evolan Pharma AB, all outside the submitted work. He is also Editor-in-Chief of JCPP Advances. Per Hove Thomsen has received a speaker’s fee from MEDICE and Takeda within the last 3 years. Trine Munk-Olsen has received a speaker’s fee from Lundbeck A/S within the last 3 years. Mette Bliddal, Malene Galle Madsen, Veerle Bergink, Chaitra Srinivas, Jacqueline M. Cohen, Isabell Brikell, and Xiaoqin Liu have no conflicts of interest to declare in relation to this work.
Ethics Approval
This study was approved by the Danish Data Protection Agency and the Danish Health Data Authority. All data have been de-identified and are not recognizable at an individual level.
Consent to Participate
Informed consent is not required for register-based studies, according to Danish law.
Consent for publication
Not applicable.
Availability of Data and Material
Access to individual-level data from Denmark is governed by Danish authorities. These include the Danish Data Protection Agency, the Danish Health Data Authority, the Ethical Committee, and Statistics Denmark. Each scientific project must be approved before initiation, and approval is granted to a specific Danish research institution. Researchers at Danish research institutions may obtain the relevant approval and data. International researchers may gain data access if governed by a Danish research institution having needed approval and data access.
Code Availability
Code can be made available upon reasonable request.
Author Contributions
All authors contributed to the study conception and design. Data management and analysis were performed by XL. The first draft of the manuscript was written by KBM and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript and agree to be accountable for the work.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Bang Madsen, K., Bliddal, M., Skoglund, C.B. et al. Attention-Deficit Hyperactivity Disorder (ADHD) Medication Use Trajectories Among Women in the Perinatal Period. CNS Drugs 38, 303–314 (2024). https://doi.org/10.1007/s40263-024-01076-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-024-01076-1