Abstract
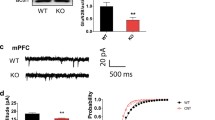
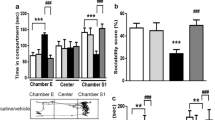
Autism spectrum disorder (ASD) is associated with a range of abnormalities characterized by deficits in socialization, communication, repetitive behaviors, and restricted interests. We have recently shown that neuronal nitric oxide synthase (nNOS) expression was decreased in the basolateral amygdala (BLA) of mice after postnatal valproic acid exposure. Neuronal activity-regulated pentraxin (Narp) could contribute to the regulation of the GluA4 2-amino-3-(5-methyl-3-oxo-1,2-oxazol-4-yl) propanoic acid (AMPA) subunits which are predominantly expressed in interneurons. However, the specific role of nNOS re-expression on excitatory neurotransmitter with relevance to ASD core symptoms in VPA-treated animals remains to be elucidated. Herein, nNOS overexpression using a lentiviral vector and L-arginine-activating PI3K-Akt-mTOR signaling can restore nNOS expression in the BLA induced by VPA. Restoration of nNOS expression in these mice was sufficient to reduce the severity of ASD-like behavioral patterns such that animals exhibited decreases in abnormal social interactions and communication, stereotyped/repetitive behaviors, and anxiety-like traits. Most strikingly, re-expression of nNOS upregulated surface expression of Narp and GluA4 in nNOS-positive interneuron as shown by immunoprecipitation and Western blotting. Whole-cell patch-clamp recordings demonstrated that restoration of nNOS had a significant enhancing effect on AMPA receptor-mediated excitatory glutamatergic synaptic neurotransmission, which was inhibited by disturbing the interaction between Narp and GluA4 in acutely dissociated BLA slices. Overall, these data offer a scientific basis for the additional study of nNOS re-expression as a promising therapeutic target by correcting AMPA receptor-mediated synaptic function in ASD and related neurodevelopmental disorders.





Similar content being viewed by others
Data Availability
All data generated for this study are available on request to the corresponding author.
References
Wang X, Gao C, Zhang Y, Hu S, Qiao Y, Zhao Z et al (2021) Overexpression of mGluR7 in the prefrontal cortex attenuates autistic behaviors in mice. Front Cell Neurosci 15:689611
Gao Y, Sun J, Cheng L, Yang Q, Li J, Hao Z et al (2022) Altered resting state dynamic functional connectivity of amygdala subregions in patients with autism spectrum disorder: a multi-site fMRI study. J Affect Disord 312:69–77
Kim S, Kim YE, Song I, Ujihara Y, Kim N, Jiang YH et al (2022) Neural circuit pathology driven by Shank3 mutation disrupts social behaviors. Cell Rep 39(10):110906
Chiola S, Napan KL, Wang Y, Lazarenko RM, Armstrong CJ, Cui J et al (2021) Defective AMPA-mediated synaptic transmission and morphology in human neurons with hemizygous SHANK3 deletion engrafted in mouse prefrontal cortex. Mol Psychiatry 26(9):4670–4686
Vyas Y, Lee K, Jung Y, Montgomery JM (2020) Influence of maternal zinc supplementation on the development of autism-associated behavioural and synaptic deficits in offspring Shank3-knockout mice. Mol Brain 13(1):110
Guo B, Chen J, Chen Q, Ren K, Feng D, Mao H et al (2019) Anterior cingulate cortex dysfunction underlies social deficits in Shank3 mutant mice. Nat Neurosci 22(8):1223–1234
Sacai H, Sakoori K, Konno K, Nagahama K, Suzuki H, Watanabe T et al (2020) Autism spectrum disorder-like behavior caused by reduced excitatory synaptic transmission in pyramidal neurons of mouse prefrontal cortex. Nat Commun 11(1):5140
Niescier RF, Lin YC (2021) The potential role of AMPA receptor trafficking in autism and other neurodevelopmental conditions. Neuroscience 479:180–191
Ganea DA, Dines M, Basu S, Lamprecht R (2015) The membrane proximal region of AMPA receptors in lateral amygdala is essential for fear memory formation. Neuropsychopharmacology 40(12):2727–2735
Wang X, Yang Z, Fang S, Zhang Y, Guo J, Gou L (2021) Declining levels of specialized synaptic surface proteins in nNOS-expressing interneurons in mice treated prenatally with valproic acid. Neurochem Res 46(7):1794–1800
Wu QL, Gao Y, Li JT, Ma WY, Chen NH (2022) The role of AMPARs composition and trafficking in synaptic plasticity and diseases. Cell Mol Neurobiol 42(8):2489–2504
Pelkey KA, Barksdale E, Craig MT, Yuan X, Sukumaran M, Vargish GA et al (2015) Pentraxins coordinate excitatory synapse maturation and circuit integration of parvalbumin interneurons. Neuron 85(6):1257–1272
Wang Z, Jin T, Le Q, Liu C, Wang X, Wang F et al (2019) Retrieval-driven hippocampal NPTX2 plasticity facilitates the extinction of cocaine-associated context memory. Biol Psychiatry 87(11):979–991
Wang X, Liu C, Wang X, Gao F, Zhan RZ (2017) Density and neurochemical profiles of neuronal nitric oxide synthase-expressing interneuron in the mouse basolateral amygdala. Brain Res 1663:106–113
Wei J-A, Han Q, Luo Z, Liu L, Cui J, Tan J et al (2022) Amygdala neural ensemble mediates mouse social investigation behaviors. Natl Sci Rev
Armstrong C, Magnuson EK, Soltesz I (2012) Neurogliaform and Ivy cells: a major family of nNOS expressing GABAergic neurons. Front Neural Circ 6:23
Li G, Stewart R, Canepari M, Capogna M (2014) Firing of hippocampal neurogliaform cells induces suppression of synaptic inhibition. J Neurosci 34(4):1280–1292
Wang X, Zhang Y, Cheng W, Gao Y, Li S (2021) Decreased excitatory drive onto hilar neuronal nitric oxide synthase expressing interneurons in chronic models of epilepsy. Brain Res 1764:147467
Wang X, Guo J, Song Y, Wang Q, Hu S, Gou L et al (2018) Decreased number and expression of nNOS-positive interneurons in basolateral amygdala in two mouse models of autism. Front Cell Neurosci 12:251–257
Dai L, Weiss RB, Dunn DM, Ramirez A, Paul S, Korenberg JR (2021) Core transcriptional networks in Williams syndrome: IGF1-PI3K-AKT-mTOR, MAPK and actin signaling at the synapse echo autism. Hum Mol Genet 30(6):411–429
Rivell A, Mattson MP (2019) Intergenerational metabolic syndrome and neuronal network hyperexcitability in autism. Trends Neurosci 42(10):709–726
Bertoni A, Schaller F, Tyzio R, Gaillard S, Santini F, Xolin M et al (2021) Oxytocin administration in neonates shapes hippocampal circuitry and restores social behavior in a mouse model of autism. Mol Psychiatry 26(12):7582–7595
Klibaite U, Kislin M, Verpeut JL, Bergeler S, Sun X, Shaevitz JW et al (2022) Deep phenotyping reveals movement phenotypes in mouse neurodevelopmental models. Mol Autism 13(1):12
Moutin E, Sakkaki S, Compan V, Bouquier N, Giona F, Areias J et al (2021) Restoring glutamate receptosome dynamics at synapses rescues autism-like deficits in Shank3-deficient mice. Mol Psychiatry 26(12):7596–7609
Wang X, Guo Z, Mei D, Zhang Y, Zhao S, Hu S et al (2022) The GluN2B-Trp373 NMDA receptor variant is associated with autism-, epilepsy-related phenotypes and reduces NMDA receptor currents in rats. Neurochem Res 47(6):1588–1597
Wang X, Mei D, Gou L, Zhao S, Gao C, Guo J et al (2023) Functional evaluation of a novel GRIN2B missense variant associated with epilepsy and intellectual disability. Neuroscience 526:107–120
Hu J, Ng YK, Chin CM, Ling EA (2008) Effects of l-arginine and N(G)-nitro-l-arginine methyl ester treatments on expression of neuronal nitric oxide synthase in the guinea-pig bladder after partial bladder outlet obstruction. Neuroscience 151(3):680–691
Dingemans AJM, Truijen KMG, Ven Svd, Bernier R, Bongers EMHF, Bouman A et al (2022) The phenotypic spectrum and genotype-phenotype correlations in 106 patients with variants in major autism gene CHD8. Transl Psychiatry 12(1):421
Yarger HA (2022) Anxiety-amygdala associations: novel insights from the first longitudinal study of autistic youth with distinct anxiety. Biol Psychiat 91(11):e41–e43
Zheng Z, Keifer J (2014) Sequential delivery of synaptic GluA1- and GluA4-containing AMPA receptors (AMPARs) by SAP97 anchored protein complexes in classical conditioning. J Biol Chem 289(15):10540–10550
Zhu JW, Zou MM, Li YF, Chen WJ, Liu JC, Chen H et al (2020) Absence of TRIM32 leads to reduced GABAergic interneuron generation and autism-like behaviors in mice via suppressing mTOR signaling. Cereb Cortex (New York, NY : 1991) 30(5):3240–3258
Gazestani VH, Pramparo T, Nalabolu S, Kellman BP, Murray S, Lopez L et al (2019) A perturbed gene network containing PI3K–AKT, RAS–ERK and WNT–β-catenin pathways in leukocytes is linked to ASD genetics and symptom severity. Nat Neurosci 22(10):1624–1634
Russo AJ (2015) Decreased phosphorylated protein kinase B (Akt) in individuals with autism associated with high epidermal growth factor receptor (EGFR) and low gamma-aminobutyric acid (GABA). Biomark Insights 10:89–94
Nicolini C, Ahn Y, Michalski B, Rho JM, Fahnestock M (2015) Decreased mTOR signaling pathway in human idiopathic autism and in rats exposed to valproic acid. Acta Neuropathol Commun 3:3
Xing X, Wu K, Dong Y, Zhou Y, Zhang J, Jiang F et al (2020) Hyperactive Akt-mTOR pathway as a therapeutic target for pain hypersensitivity in Cntnap2-deficient mice. Neuropharmacology 165:107816
Shih PY, Fang YL, Shankar S, Lee SP, Hu HT, Chen H et al (2022) Phase separation and zinc-induced transition modulate synaptic distribution and association of autism-linked CTTNBP2 and SHANK3. Nat Commun 13(1):2664
Mishra A, Singla R, Kumar R, Sharma A, Joshi R, Sarma P et al (2022) Granulocyte colony-stimulating factor improved core symptoms of autism spectrum disorder via modulating glutamatergic receptors in the prefrontal cortex and hippocampus of rat brains. ACS Chem Neurosci 13:2942–2961
Barreto LE, Morales M (2016) The PI3K signaling pathway as a pharmacological target in autism related disorders and schizophrenia. Mol Cell Ther 4:2
Cupolillo D, Hoxha E, Faralli A (2016) Autistic-like traits and cerebellar dysfunction in Purkinje cell PTEN knock-out mice. Neuropsychopharmacology. 41(6):1457–1466
Mellios N, Feldman DA, Sheridan SD, Ip JPK, Kwok S, Amoah SK et al (2018) MeCP2-regulated miRNAs control early human neurogenesis through differential effects on ERK and AKT signaling. Molecular psychiatry. 23(4):1051–1065
Zhu LJ, Xu C, Ren J, Chang L, Zhu XH, Sun N et al (2020) Dentate nNOS accounts for stress-induced 5-HT1A receptor deficiency: implication in anxiety behaviors. CNS Neurosci Ther 26(4):453–464
Zhou QG, Zhu XH, Nemes AD, Zhu DY (2018) Neuronal nitric oxide synthase and affective disorders. IBRO Rep 5:116–132
Chang MC, Park JM, Pelkey KA, Grabenstatter HL, Xu D, Linden DJ et al (2010) Narp regulates homeostatic scaling of excitatory synapses on parvalbumin-expressing interneurons. Nat Neurosci 13(9):1090–1097
Heinrich IA, Freitas AE, Wolin IAV, Nascimento APM, Walz R, Rodrigues ALS et al (2021) neuronal activity regulated pentraxin (narp) and GluA4 subunit of AMPA receptor may be targets for fluoxetine modulation. Metab Brain Dis 36(4):711–722
Mei Y, Monteiro P, Zhou Y, Kim JA, Gao X, Fu Z et al (2016) Adult restoration of Shank3 expression rescues selective autistic-like phenotypes. Nature 530(7591):481–484
Cao W, Lin S, Xia QQ, Du YL, Yang Q, Zhang MY et al (2018) Gamma oscillation dysfunction in mPFC leads to social deficits in neuroligin 3 R451C knockin mice. Neuron 97(6):1253–1260
Kim JW, Park K, Kang RJ, Gonzales ELT, Kim DG, Oh HA et al (2019) Pharmacological modulation of AMPA receptor rescues social impairments in animal models of autism. Neuropsychopharmacology 44(2):314–332
Funding
We gratefully acknowledge the sponsorship from the National Natural Science Foundation of China (81901387), the Scientific and Technological Project of Henan (232102311006, 212102310034, 232102310077), the Medical Science and Technique Program of Henan (LHGJ20210632), the Natural Science Foundation of Henan (232300421086), the Henan Neural Development Engineering Research Center for Children Foundation (SG202202), and the Joint Fund of Henan Provincial Science and Technology Research (225200810114).
Author information
Authors and Affiliations
Contributions
XW designed the research and wrote the manuscript. KZ conducted the viral infusion surgeries. SL and SH performed the behavioral characterization and analyzed the data. YD, DM, and CG carried out western blotting. XW performed electrophysiological experiments and analyzed the data as well. XW and YZ supervised all aspects of the research. All authors have read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
The animal study was reviewed and approved by the Institutional Animal Ethics Committee of Zhengzhou University, following the NIH Guidelines for the Care and Use of Laboratory Animals.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, X., Zhang, Y., Luo, S. et al. Restoration of nNOS Expression Rescues Autistic-Like Phenotypes Through Normalization of AMPA Receptor-Mediated Neurotransmission. Mol Neurobiol (2024). https://doi.org/10.1007/s12035-024-03997-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12035-024-03997-w