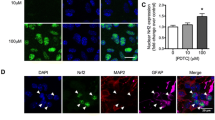
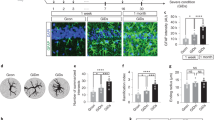
Abstract
Oxidative stress is involved in the pathogenesis of Alzheimer’s disease (AD), which is linked to reactive oxygen species (ROS), lipid peroxidation, and neurotoxicity. Emerging evidence suggests a role of nuclear factor (erythroid-derived 2)-like 2 (Nrf2), a major source of antioxidant response elements in AD. The molecular mechanism of oxidative stress and ferroptosis in astrocytes in AD is not yet fully understood. Here, we aimed to investigate the mechanism by which Nrf2 regulates the ferroptosis of astrocytes in AD. We found decreased expression of Nrf2 and upregulated expression of the ROS marker NADPH oxidase 4 (NOX4) in the frontal cortex from patients with AD and in the cortex of 3×Tg mice compared to wildtype mice. We demonstrated that Nrf2 deficiency led to ferroptosis-dependent oxidative stress-induced ROS with downregulated heme oxygenase-1 and glutathione peroxidase 4 and upregulated cystine glutamate expression. Moreover, Nrf2 deficiency increased lipid peroxidation, DNA oxidation, and mitochondrial fragmentation in mouse astrocytes (mAS, M1800-57). In conclusion, these results suggest that Nrf2 deficiency promotes ferroptosis of astrocytes involving oxidative stress in AD.








Similar content being viewed by others
Data Availability
Data will be made available upon reasonable request.
References
De Strooper B, Karran E (2016) The cellular phase of Alzheimer’s disease. Cell 164(4):603–615
Wilson DM 3rd, Cookson MR, Van Den Bosch L et al (2023) Hallmarks of neurodegenerative diseases. Cell 186(4):693–714
Brandebura AN, Paumier A, Onur TS et al (2023) Astrocyte contribution to dysfunction, risk and progression in neurodegenerative disorders. Nat Rev Neurosci 24(1):23–39
Escartin C, Galea E, Lakatos A et al (2021) Reactive astrocyte nomenclature, definitions, and future directions. Nat Neurosci 24(3):312–325
Hasel P, Rose IVL, Sadick JS et al (2021) Neuroinflammatory astrocyte subtypes in the mouse brain. Nat Neurosci 24(10):1475–1487
Habib N, McCabe C, Medina S et al (2020) Disease-associated astrocytes in Alzheimer’s disease and aging. Nat Neurosci 23(6):701–706
Kecheliev V, Spinelli F, Herde A et al (2022) Evaluation of cannabinoid type 2 receptor expression and pyridine-based radiotracers in brains from a mouse model of Alzheimer’s disease. Front Aging Neurosci 14:1018610
Rodriguez-Vieitez E, Ni R, Gulyás B et al (2015) Astrocytosis precedes amyloid plaque deposition in Alzheimer APPswe transgenic mouse brain: a correlative positron emission tomography and in vitro imaging study. Eur J Nucl Med Mol Imaging 42(7):1119–1132
Butterfield DA, Halliwell B (2019) Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nat Rev Neurosci 20(3):148–160
Cheignon C, Tomas M, Bonnefont-Rousselot D et al (2018) Oxidative stress and the amyloid beta peptide in Alzheimer’s disease. Redox Biol 14:450–464
Cioffi F, Adam RHI, Bansal R et al (2021) A review of oxidative stress products and related genes in early Alzheimer’s disease. J Alzheim Dis : JAD 83(3):977–1001
Ionescu-Tucker A, Cotman CW (2021) Emerging roles of oxidative stress in brain aging and Alzheimer’s disease. Neurobiol Aging 107:86–95
Nunomura A, Perry G (2020) RNA and oxidative stress in Alzheimer’s disease: focus on microRNAs. Oxid Med Cell Longev 2020:2638130
Bonda DJ, Wang X, Lee HG et al (2014) Neuronal failure in Alzheimer’s disease: a view through the oxidative stress looking-glass. Neurosci Bull 30(2):243–252
Perez Ortiz JM, Swerdlow RH (2019) Mitochondrial dysfunction in Alzheimer’s disease: role in pathogenesis and novel therapeutic opportunities. Br J Pharmacol 176(18):3489–3507
Wood H (2020) Mitochondrial dysfunction manifests in the early stages of Alzheimer disease. Nat Rev Neurol 16(5):242
Chiang MC, Nicol CJB (2022) GSH-AuNP anti-oxidative stress, ER stress and mitochondrial dysfunction in amyloid-beta peptide-treated human neural stem cells. Free Radical Biol Med 187:185–201
Cheignon C, Jones M, Atrián-Blasco E et al (2017) Identification of key structural features of the elusive Cu-Aβ complex that generates ROS in Alzheimer’s disease. Chem Sci 8(7):5107–5118
Gleason A, Bush AI (2021) Iron and ferroptosis as therapeutic targets in Alzheimer’s disease. Neurotherap :J Am Soc Exp NeuroTherap 18(1):252–264
Li J, Cao F, Yin HL et al (2020) Ferroptosis: past, present and future. Cell Death Dis 11(2):88
Yan HF, Zou T, Tuo QZ et al (2021) Ferroptosis: mechanisms and links with diseases. Signal Transduct Target Ther 6(1):49
Jakaria M, Belaidi AA, Bush AI et al (2021) Ferroptosis as a mechanism of neurodegeneration in Alzheimer’s disease. J Neurochem 159(5):804–825
Galluzzi L, Vitale I, Aaronson SA et al (2018) Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ 25(3):486–541
Tang D, Chen X, Kang R et al (2021) Ferroptosis: molecular mechanisms and health implications. Cell Res 31(2):107–125
Zhang J, Wang X, Guan B et al (2023) Qing-Xin-Jie-Yu Granule inhibits ferroptosis and stabilizes atherosclerotic plaques by regulating the GPX4/xCT signaling pathway. J Ethnopharmacol 301:115852
Wang Z, Ding Y, Wang X et al (2018) Pseudolaric acid B triggers ferroptosis in glioma cells via activation of Nox4 and inhibition of xCT. Cancer Lett 428:21–33
Zhang Z, Tang J, Song J et al (2022) Elabela alleviates ferroptosis, myocardial remodeling, fibrosis and heart dysfunction in hypertensive mice by modulating the IL-6/STAT3/GPX4 signaling. Free Radical Biol Med 181:130–142
Bersuker K, Hendricks JM, Li Z et al (2019) The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature 575(7784):688–692
Ashraf A, Jeandriens J, Parkes HG et al (2020) Iron dyshomeostasis, lipid peroxidation and perturbed expression of cystine/glutamate antiporter in Alzheimer’s disease: evidence of ferroptosis. Redox Biol 32:101494
Friedmann Angeli JP, Schneider M, Proneth B et al (2014) Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat Cell Biol 16(12):1180–1191
Skouta R, Dixon SJ, Wang J et al (2014) Ferrostatins inhibit oxidative lipid damage and cell death in diverse disease models. J Am Chem Soc 136(12):4551–4556
Park MW, Cha HW, Kim J et al (2021) NOX4 promotes ferroptosis of astrocytes by oxidative stress-induced lipid peroxidation via the impairment of mitochondrial metabolism in Alzheimer’s diseases. Redox Biol 41:101947
Johnson J, Mercado-Ayon E, Mercado-Ayon Y et al (2021) Mitochondrial dysfunction in the development and progression of neurodegenerative diseases. Arch Biochem Biophys 702:108698
Dinkova-Kostova AT, Kostov RV, Kazantsev AG (2018) The role of Nrf2 signaling in counteracting neurodegenerative diseases. FEBS J 285(19):3576–3590
Osama A, Zhang J, Yao J et al (2020) Nrf2: a dark horse in Alzheimer’s disease treatment. Ageing Res Rev 64:101206
Qu Z, Sun J, Zhang W et al (2020) Transcription factor NRF2 as a promising therapeutic target for Alzheimer’s disease. Free Radical Biol Med 159:87–102
Lipton SA, Rezaie T, Nutter A et al (2016) Therapeutic advantage of pro-electrophilic drugs to activate the Nrf2/ARE pathway in Alzheimer’s disease models. Cell Death Dis 7(12):e2499
Adlimoghaddam A, Odero GG, Glazner G et al (2021) Nilotinib improves bioenergetic profiling in brain astroglia in the 3xTg mouse model of Alzheimer’s disease. Aging Dis 12(2):441–465
Esteras N, Dinkova-Kostova AT, Abramov AY (2016) Nrf2 activation in the treatment of neurodegenerative diseases: a focus on its role in mitochondrial bioenergetics and function. Biol Chem 397(5):383–400
Johnson DA, Johnson JA (2015) Nrf2–a therapeutic target for the treatment of neurodegenerative diseases. Free Radical Biol Med 88(Pt B):253–267
Sotolongo K, Ghiso J, Rostagno A (2020) Nrf2 activation through the PI3K/GSK-3 axis protects neuronal cells from Aβ-mediated oxidative and metabolic damage. Alzheim Res Ther 12(1):13
Bahn G, Park JS, Yun UJ et al (2019) NRF2/ARE pathway negatively regulates BACE1 expression and ameliorates cognitive deficits in mouse Alzheimer’s models. Proc Natl Acad Sci USA 116(25):12516–12523
Oddo S, Caccamo A, Shepherd JD et al (2003) Triple-transgenic model of Alzheimer’s disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron 39(3):409–421
Ni R, Röjdner J, Voytenko L et al (2021) In vitro characterization of the regional binding distribution of amyloid PET tracer florbetaben and the glia tracers deprenyl and PK11195 in autopsy Alzheimer’s brain tissue. J Alzheim Dis: JAD 80(4):1723–1737
Ni R, Gillberg PG, Bogdanovic N et al (2017) Amyloid tracers binding sites in autosomal dominant and sporadic Alzheimer’s disease. Alzheim Demen : J Alzheim Assoc 13(4):419–430
Lai C, Chen Z, Ding Y et al (2022) Rapamycin attenuated zinc-induced tau phosphorylation and oxidative stress in rats: involvement of dual mTOR/p70S6K and Nrf2/HO-1 pathways. Front Immunol 13:782434
Wang L, Tang Z, Deng Y et al (2023) Myricetin protected against Aβ oligomer-induced synaptic impairment, mitochondrial function and oxidative stress in SH-SY5Y cells via ERK1/2/GSK-3β pathways. bioRxiv 2023.01.12.523781
Tang Z, Guo M, Peng Y et al (2022) Quercetin reduces APP expression, oxidative stress and mitochondrial dysfunction in the N2a/APPswe cells via ERK1/2 and AKT pathways. bioRxiv 2022.09.18.508406
Chen Q, Lai C, Chen F et al (2022) Emodin protects SH-SY5Y cells against zinc-induced synaptic impairment and oxidative stress through the ERK1/2 pathway. Front Pharmacol 13:821521
Li Y, Zhao T, Li J et al (2022) Oxidative stress and 4-hydroxy-2-nonenal (4-HNE): implications in the pathogenesis and treatment of aging-related diseases. J Immunol Res 2022:2233906
Angelova PR, Abramov AY (2018) Role of mitochondrial ROS in the brain: from physiology to neurodegeneration. FEBS Lett 592(5):692–702
Tönnies E, Trushina E (2017) Oxidative stress, synaptic dysfunction, and Alzheimer’s disease. J Alzheim Dis: JAD 57(4):1105–1121
Resende R, Moreira PI, Proença T et al (2008) Brain oxidative stress in a triple-transgenic mouse model of Alzheimer disease. Free Radical Biol Med 44(12):2051–2057
Matsumura A, Emoto MC, Suzuki S et al (2015) Evaluation of oxidative stress in the brain of a transgenic mouse model of Alzheimer disease by in vivo electron paramagnetic resonance imaging. Free Radical Biol Med 85:165–173
Stefanatos R, Sanz A (2018) The role of mitochondrial ROS in the aging brain. FEBS Lett 592(5):743–758
Pareek V, Nath B, Roy PK (2019) Role of neuroimaging modality in the assessment of oxidative stress in brain: a comprehensive review. CNS Neurol Disord: Drug Targets 18(5):372–381
Cobley JN, Fiorello ML, Bailey DM (2018) 13 reasons why the brain is susceptible to oxidative stress. Redox Biol 15:490–503
Aborode AT, Pustake M, Awuah WA et al (2022) Targeting oxidative stress mechanisms to treat Alzheimer’s and Parkinson’s disease: a critical review. Oxid Med Cell Longev 2022:7934442
Anwar MM (2022) Oxidative stress-A direct bridge to central nervous system homeostatic dysfunction and Alzheimer’s disease. Cell Biochem Funct 40(1):17–27
Francesca F, Caitlin A, Sarah L et al (2022) Antroquinonol administration in animal preclinical studies for Alzheimer’s disease (AD): a new avenue for modifying progression of AD pathophysiology. Brain, Behav Immun Health 21:100435
Tao W, Yu L, Shu S et al (2021) miR-204-3p/Nox4 mediates memory deficits in a mouse model of Alzheimer’s disease. Mole Ther : J Am Soc Gene Ther 29(1):396–408
Chun H, Im H, Kang YJ et al (2020) Severe reactive astrocytes precipitate pathological hallmarks of Alzheimer’s disease via H(2)O(2)(-) production. Nat Neurosci 23(12):1555–1566
Guillemaud O, Ceyzériat K, Saint-Georges T et al (2020) Complex roles for reactive astrocytes in the triple transgenic mouse model of Alzheimer disease. Neurobiol Aging 90:135–146
Jiwaji Z, Tiwari SS, Avilés-Reyes RX et al (2022) Reactive astrocytes acquire neuroprotective as well as deleterious signatures in response to Tau and Aß pathology. Nat Commun 13(1):135
Bellaver B, Povala G, Ferreira PCL, et al. Astrocyte reactivity influences amyloid-β effects on tau pathology in preclinical Alzheimer’s disease. Nature medicine. 2023.
Xu S, Wu B, Zhong B et al (2021) Naringenin alleviates myocardial ischemia/reperfusion injury by regulating the nuclear factor-erythroid factor 2-related factor 2 (Nrf2) /System xc-/ glutathione peroxidase 4 (GPX4) axis to inhibit ferroptosis. Bioengineered 12(2):10924–10934
Feng Y, Wang X (2012) Antioxidant therapies for Alzheimer’s disease. Oxid Med Cell Longev 2012:472932
Zeng K, Yu X, Mahaman YAR et al (2022) Defective mitophagy and the etiopathogenesis of Alzheimer’s disease. Transl Neurodegen 11(1):32
Su B, Wang X, Nunomura A et al (2008) Oxidative stress signaling in Alzheimer’s disease. Curr Alzheimer Res 5(6):525–532
Dixon SJ, Patel DN, Welsch M et al (2014) Pharmacological inhibition of cystine-glutamate exchange induces endoplasmic reticulum stress and ferroptosis. eLife 3:e02523
Chen X, Yu C, Kang R et al (2021) Cellular degradation systems in ferroptosis. Cell Death Differ 28(4):1135–1148
Dixon SJ, Lemberg KM, Lamprecht MR et al (2012) Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149(5):1060–1072
Li K, Reichmann H (2016) Role of iron in neurodegenerative diseases. J Neural Transm 123(4):389–399
Tu H, Tang LJ, Luo XJ et al (2021) Insights into the novel function of system Xc- in regulated cell death. Eur Rev Med Pharmacol Sci 25(3):1650–1662
Stockwell BR, Friedmann Angeli JP, Bayir H et al (2017) Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell 171(2):273–285
Conrad M, Kagan VE, Bayir H et al (2018) Regulation of lipid peroxidation and ferroptosis in diverse species. Genes Dev 32(9–10):602–619
Liu M, Kong XY, Yao Y et al (2022) The critical role and molecular mechanisms of ferroptosis in antioxidant systems: a narrative review. Ann Transl Med 10(6):368
Su LJ, Zhang JH, Gomez H et al (2019) Reactive oxygen species-induced lipid peroxidation in apoptosis, autophagy, and ferroptosis. Oxid Med Cell Longev 2019:5080843
Ursini F, Maiorino M (2020) Lipid peroxidation and ferroptosis: The role of GSH and GPx4. Free Radical Biol Med 152:175–185
Park E, Chung SW (2019) ROS-mediated autophagy increases intracellular iron levels and ferroptosis by ferritin and transferrin receptor regulation. Cell Death Dis 10(11):822
Mecocci P, Boccardi V, Cecchetti R et al (2018) A long journey into aging, brain aging, and Alzheimer’s disease following the oxidative stress tracks. J Alzheim Dis: JAD 62(3):1319–1335
Nunomura A, Perry G, Pappolla MA et al (1999) RNA oxidation is a prominent feature of vulnerable neurons in Alzheimer’s disease. J Neurosci :J Soc Neurosci 19(6):1959–1964
Gómez-Pineda VG, Torres-Cruz FM, Vivar-Cortés CI et al (2018) Neurotrophin-3 restores synaptic plasticity in the striatum of a mouse model of Huntington’s disease. CNS Neurosci Ther 24(4):353–363
Ren P, Chen J, Li B et al (2020) Nrf2 ablation promotes Alzheimer’s disease-like pathology in APP/PS1 transgenic mice: the role of neuroinflammation and oxidative stress. Oxid Med Cell Longev 2020:3050971
Branca C, Ferreira E, Nguyen TV et al (2017) Genetic reduction of Nrf2 exacerbates cognitive deficits in a mouse model of Alzheimer’s disease. Hum Mol Genet 26(24):4823–4835
Uruno A, Matsumaru D, Ryoke R, et al. Nrf2 suppresses oxidative stress and inflammation in app knock-in Alzheimer’s disease model mice. Molecular and cellular biology. 2020;40(6).
Abdalkader M, Lampinen R, Kanninen KM et al (2018) Targeting Nrf2 to suppress ferroptosis and mitochondrial dysfunction in neurodegeneration. Front Neurosci 12:466
Liddell JR. Are astrocytes the predominant cell type for activation of Nrf2 in aging and neurodegeneration? Antioxidants. 2017;6(3).
Wang W, Zhao F, Ma X et al (2020) Mitochondria dysfunction in the pathogenesis of Alzheimer’s disease: recent advances. Mol Neurodegener 15(1):30
Sorrentino V, Romani M, Mouchiroud L et al (2017) Enhancing mitochondrial proteostasis reduces amyloid-β proteotoxicity. Nature 552(7684):187–193
Ashleigh T, Swerdlow RH, Beal MF (2023) The role of mitochondrial dysfunction in Alzheimer’s disease pathogenesis. Alzheim Dement 19(1):333–342
Dhapola R, Sarma P, Medhi B et al (2022) Recent advances in molecular pathways and therapeutic implications targeting mitochondrial dysfunction for Alzheimer’s disease. Mol Neurobiol 59(1):535–555
Zhu CC, Fu SY, Chen YX et al (2020) Advances in drug therapy for Alzheimer’s disease. Curr Med Sci 40(6):999–1008
Jackson JG, Robinson MB (2018) Regulation of mitochondrial dynamics in astrocytes: Mechanisms, consequences, and unknowns. Glia 66(6):1213–1234
Joshi AU, Minhas PS, Liddelow SA et al (2019) Fragmented mitochondria released from microglia trigger A1 astrocytic response and propagate inflammatory neurodegeneration. Nat Neurosci 22(10):1635–1648
Hung CH, Cheng SS, Cheung YT et al (2018) A reciprocal relationship between reactive oxygen species and mitochondrial dynamics in neurodegeneration. Redox Biol 14:7–19
Dematteis G, Vydmantaitė G, Ruffinatti FA et al (2020) Proteomic analysis links alterations of bioenergetics, mitochondria-ER interactions and proteostasis in hippocampal astrocytes from 3xTg-AD mice. Cell Death Dis 11(8):645
Li Q, Han X, Lan X et al (2017) Inhibition of neuronal ferroptosis protects hemorrhagic brain. JCI insight 2(7):e90777
Liddell JR, White AR (2018) Nexus between mitochondrial function, iron, copper and glutathione in Parkinson’s disease. Neurochem Int 117:126–138
Joshi G, Gan KA, Johnson DA et al (2015) Increased Alzheimer’s disease-like pathology in the APP/ PS1ΔE9 mouse model lacking Nrf2 through modulation of autophagy. Neurobiol Aging 36(2):664–679
Liu Z, Han K, Huo X et al (2020) Nrf2 knockout dysregulates iron metabolism and increases the hemolysis through ROS in aging mice. Life Sci 255:117838
Lai C, Chen Q, Ding Y et al (2020) Emodin protected against synaptic impairment and oxidative stress induced by fluoride in SH-SY5Y cells by modulating ERK1/2/Nrf2/HO-1 pathway. Environ Toxicol 35(9):922–929
Sumien N, Cunningham JT, Davis DL, et al. Neurodegenerative disease: roles for sex, hormones, and oxidative stress. Endocrinology. 2021;162(11).
Funding
This work was supported by the Chinese National Natural Science Foundation (81960265, 82260263), the China Postdoctoral Science Foundation (2020M683659XB), the Foundation for Guizhou Provincial Science and Technology projects ([2020]1Y354 and [2023]232), the Department of Education of Guizhou Province [Nos. KY (2021)313], the Scientific Research Project of Guizhou Medical University (J[48]), and the Foundation for Science and Technology projects in Guiyang ([2019]9–2-7).
Author information
Authors and Affiliations
Contributions
Conceptualization ZT, RN, and XLQ; data curation, investigation, formal analysis: ZYC, ZT, and YX; supervision, resources, project administration: RN and ZT; Original draft: XLQ, ZYC, RN, and ZT; review and editing: all authors.
Corresponding authors
Ethics declarations
Ethics Approval
The study of human brain tissues was conducted according to the principles of the Declaration of Helsinki and subsequent revisions and ethical permission obtained from the regional human ethics committee in Guiyang Hospital (approval No. 2022290) and the medical ethics committee of the VU Medical Center for NBB tissue. And all the animal experiments were performed in accordance with guidelines under the approval of the Animal Protection and Use Committee of Guizhou Medical University (approval No. 2201640).
Consent to Participate
Informed consent was obtained from all individual participants included in the study.
Consent for Publication
All authors have given final approval of the version and agreed with the publication of this study here.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tang, Z., Chen, Z., Guo, M. et al. NRF2 Deficiency Promotes Ferroptosis of Astrocytes Mediated by Oxidative Stress in Alzheimer’s Disease. Mol Neurobiol (2024). https://doi.org/10.1007/s12035-024-04023-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12035-024-04023-9