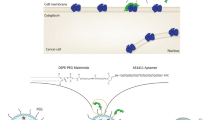
Abstract
Colorectal cancer (CRC) is the fourth most common cancer in the world, with the second highest incidence rate after lung cancer. Oxaliplatin (OXA) is a broad-spectrum anti-tumor agent with significant therapeutic efficacy in colorectal cancer, and as a divalent platinum analog, it is not selective in its distribution in the body and has systemic toxicity with continued use. Interleukin-12 (IL12) is an immunostimulatory cytokine with cytokine monotherapy that has made advances in the fight against cancer, limiting the clinical use of cytokines due to severe toxicity. Here, we introduced a long alkyl chain and N-methyl-2,2-diaminodiethylamine to the ligand of OXA to obtain OXA-LIP, which effectively reduces its toxicity and improves the uptake of the drug by tumor cells. We successfully constructed IL12 mRNA and used LNPs to deliver IL12 mRNA, and in vivo pharmacodynamic studies demonstrated that OXA-LIP combined with IL12 mRNA had better tumor inhibition and higher biosafety. In addition, it was investigated by pharmacokinetic experiments that the OXA-LIP drug could accumulate in nude mice at the tumor site, which prolonged the half-life and enhanced the anti-tumor efficiency of OXA. It is hoped that these results will provide an important reference for the subsequent research and development of OXA-LIP with IL12 mRNA, as well as provide new therapeutic approaches for the treatment of colon cancer.
Graphical Abstract












Similar content being viewed by others
Availability of data and materials
Not applicable.
References
Dekker E, Tanis PJ, Vleugels JLA, et al. Colorectal cancer. Lancet. 2019;394(10207):1467–1480. https://doi.org/10.1016/s0140-6736(19)32319-0.
Cao W, Chen HD, Yu YW, et al. Changing profiles of cancer burden worldwide and in China: a secondary analysis of the global cancer statistics 2020. Chin Med J (Engl). 2021;134(7):783–791. https://doi.org/10.1097/cm9.0000000000001474.
Xia C, Dong X, Li H, et al. Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin Med J (Engl). 2022;135(5):584–590. https://doi.org/10.1097/cm9.0000000000002108.
Chen L, Han X. Anti-PD-1/PD-L1 therapy of human cancer: past, present, and future. J Clin Invest. 2015;125(9):3384–91. https://doi.org/10.1172/jci80011.
Taube JM, Klein A, Brahmer JR, et al. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res. 2014;20(19):5064–74. https://doi.org/10.1158/1078-0432.Ccr-13-3271.
Zhang YX, Zhao YY, Shen J, et al. Nanoenabled modulation of acidic tumor microenvironment reverses anergy of infiltrating T cells and potentiates anti-PD-1 therapy. Nano Lett. 2019;19(5):2774–2783. https://doi.org/10.1021/acs.nanolett.8b04296.
Zhou F, Feng B, Yu H, et al. Tumor microenvironment-activatable prodrug vesicles for nanoenabled cancer chemoimmunotherapy combining immunogenic cell death induction and CD47 blockade. Adv Mater. 2019;31(14):e1805888. https://doi.org/10.1002/adma.201805888.
Wang G, Yang B, Fu Z, et al. Efficacy and safety of oxaliplatin-based regimen versus cisplatin-based regimen in the treatment of gastric cancer: a meta-analysis of randomized controlled trials. Int J Clin Oncol. 2019;24(6):614–623. https://doi.org/10.1007/s10147-019-01425-x.
Perego P, Robert J. Oxaliplatin in the era of personalized medicine: from mechanistic studies to clinical efficacy. Cancer Chemother Pharmacol. 2016;77(1):5–18. https://doi.org/10.1007/s00280-015-2901-x.
Fu Y, Kong Y, Li X, et al. Novel Pt(IV) prodrug self-assembled nanoparticles with enhanced blood circulation stability and improved antitumor capacity of oxaliplatin for cancer therapy. Drug Deliv. 2023;30(1):2171158. https://doi.org/10.1080/10717544.2023.2171158.
Mu M, Zhan J, Dai X, et al. Research progress of azido-containing Pt(IV) antitumor compounds. Eur J Med Chem. 2022;227:113927. https://doi.org/10.1016/j.ejmech.2021.113927.
Venkatesh V, Sadler PJ. Platinum(IV) prodrugs. Met Ions Life Sci. 2018;18:69–108. https://doi.org/10.1515/9783110470734-009.
Liu X, Barth MC, Cseh K, et al. Oxoplatin-based Pt(IV) Lipoate complexes and their biological activity. Chem Biodivers. 2022;19(10):e202200695. https://doi.org/10.1002/cbdv.202200695.
Liu X, Wenisch D, Dahlke P, et al. Multi-action platinum(IV) prodrugs conjugated with COX-inhibiting NSAIDs. Eur J Med Chem. 2023;257:115515. https://doi.org/10.1016/j.ejmech.2023.115515.
Chapman RW, Corboz MR, Malinin VS, et al. An overview of the biology of a long-acting inhaled treprostinil prodrug. Pulm Pharmacol Ther. 2020;65:102002. https://doi.org/10.1016/j.pupt.2021.102002.
Colombo MP, Trinchieri G. Interleukin-12 in anti-tumor immunity and immunotherapy. Cytokine Growth Factor Rev. 2002;13(2):155–68. https://doi.org/10.1016/s1359-6101(01)00032-6.
Del Vecchio M, Bajetta E, Canova S, et al. Interleukin-12: biological properties and clinical application. Clin Cancer Res. 2007;13(16):4677–85. https://doi.org/10.1158/1078-0432.Ccr-07-0776.
Landoni E, Woodcock MG, Barragan G, et al. IL-12 reprograms CAR-expressing natural killer T cells to long-lived Th1-polarized cells with potent antitumor activity. Nat Commun. 2024;15(1):89. https://doi.org/10.1038/s41467-023-44310-y.
Cirella A, Berraondo P, Di Trani CA, et al. Interleukin-12 message in a bottle. Clin Cancer Res. 2020;26(23):6080–6082. https://doi.org/10.1158/1078-0432.Ccr-20-3250.
Wang Q, Cheng F, Ma TT, et al. Interleukin-12 inhibits the hepatocellular carcinoma growth by inducing macrophage polarization to the M1-like phenotype through downregulation of Stat-3. Mol Cell Biochem. 2016;415(1–2):157–68. https://doi.org/10.1007/s11010-016-2687-0.
Hewitt SL, Bailey D, Zielinski J, et al. Intratumoral IL12 mRNA therapy promotes TH1 transformation of the tumor microenvironment. Clin Cancer Res. 2020;26(23):6284–6298. https://doi.org/10.1158/1078-0432.Ccr-20-0472.
Jung HN, Lee SY, Lee S, et al. Lipid nanoparticles for delivery of RNA therapeutics: current status and the role of in vivo imaging. Theranostics. 2022;12(17):7509–7531. https://doi.org/10.7150/thno.77259.
Liu JQ, Zhang C, Zhang X, et al. Intratumoral delivery of IL-12 and IL-27 mRNA using lipid nanoparticles for cancer immunotherapy. J Control Release. 2022;345:306–313. https://doi.org/10.1016/j.jconrel.2022.03.021.
Wang C, Zhang Y, Dong Y. Lipid nanoparticle-mRNA formulations for therapeutic applications. Acc Chem Res. 2021;54(23):4283–4293. https://doi.org/10.1021/acs.accounts.1c00550.
Tugues S, Burkhard SH, Ohs I, et al. New insights into IL-12-mediated tumor suppression. Cell Death Differ. 2015;22(2):237–46. https://doi.org/10.1038/cdd.2014.134.
Feng B, Zhou FY, Xu ZA, et al. Versatile prodrug nanoparticles for acid-triggered precise imaging and organelle-specific combination cancer therapy. Adv Funct Mater. 2016;26(41):7431–7442. https://doi.org/10.1002/adfm.201602963.
Lang T, Li N, Zhang J, et al. Prodrug-based nano-delivery strategy to improve the antitumor ability of carboplatin in vivo and in vitro. Drug Deliv. 2021;28(1):1272–1280. https://doi.org/10.1080/10717544.2021.1938754.
Zhang Y, Li D, Shen Y, et al. Immunization with a novel mRNA vaccine, TGGT1_216200 mRNA-LNP, prolongs survival time in BALB/c mice against acute toxoplasmosis. Front Immunol. 2023;14:1161507. https://doi.org/10.3389/fimmu.2023.1161507.
Pardi N, Hogan MJ, Porter FW, et al. mRNA vaccines - a new era in vaccinology. Nat Rev Drug Discov. 2018;17(4):261–279. https://doi.org/10.1038/nrd.2017.243.
Huysmans H, De Temmerman J, Zhong Z, et al. Improving the repeatability and efficacy of intradermal electroporated self-replicating mRNA. Mol Ther Nucleic Acids. 2019;17:388–395. https://doi.org/10.1016/j.omtn.2019.06.011.
Cu Y, Broderick KE, Banerjee K, et al. Enhanced delivery and potency of self-amplifying mRNA vaccines by electroporation in situ. Vaccines (Basel). 2013;1(3):367–83. https://doi.org/10.3390/vaccines1030367.
Li B, Cai M, Lin L, et al. MRI-visible and pH-sensitive micelles loaded with doxorubicin for hepatoma treatment. Biomater Sci. 2019;7(4):1529–1542. https://doi.org/10.1039/c8bm01501e.
Wang XN, Li Y, Meng L, et al. Evaluation of influence of telmisartan on the pharmacokinetics and tissue distribution of canagliflozin in rats and mice. Ann Palliat Med. 2021;10(3):3086–3096. https://doi.org/10.21037/apm-21-65.
Li T, Qian Y, Li H, et al. Combination of serum lipids and cancer antigens as a novel marker for colon cancer diagnosis. Lipids Health Dis. 2018;17(1):261. https://doi.org/10.1186/s12944-018-0911-5.
Zhang X, Qin H, Tan X, et al. Predictive value of monocyte to high-density lipoprotein cholesterol ratio and tumor markers in colorectal cancer and their relationship with clinicopathological characteristics. World J Surg Oncol. 2023;21(1):200. https://doi.org/10.1186/s12957-023-03079-6.
Riedl JM, Posch F, Prager G, et al. The AST/ALT (De Ritis) ratio predicts clinical outcome in patients with pancreatic cancer treated with first-line nab-paclitaxel and gemcitabine: post hoc analysis of an Austrian multicenter, noninterventional study. Ther Adv Med Oncol. 2020;12:1758835919900872. https://doi.org/10.1177/1758835919900872.
Bahia MS, Silakari O. Tumor necrosis factor alpha converting enzyme: an encouraging target for various inflammatory disorders. Chem Biol Drug Des. 2010;75(5):415–43. https://doi.org/10.1111/j.1747-0285.2010.00950.x.
van Horssen R, Ten Hagen TL, Eggermont AM. TNF-alpha in cancer treatment: molecular insights, antitumor effects, and clinical utility. Oncologist. 2006;11(4):397–408. https://doi.org/10.1634/theoncologist.11-4-397.
Margraf A, Ludwig N, Zarbock A, et al. Systemic inflammatory response syndrome after surgery: mechanisms and protection. Anesth Analg. 2020;131(6):1693–1707. https://doi.org/10.1213/ane.0000000000005175.
Funding
This work was supported by National Natural Science Foundation of China (32070927), Natural Science Foundation of Shandong Province (ZR2023MC121), and Science and Technology Innovation Development Planning of Yantai (2023JCYJ060, 2023JCYJ064).
Author information
Authors and Affiliations
Contributions
Hui Liu: conceptualization, writing — original draft, writing — review and editing. Yating Du: writing — original draft, writing — review and editing. Yuanlei Fu: conceptualization, writing — review and editing, supervision. Haiqiang Cao: conceptualization, writing — review and editing, supervision. Jianpeng Yin: investigation, resources, visualization, writing — review and editing. Desheng Zhan: writing — review and editing. Wenjun Yu: writing — review and editing. Yan Li: writing — review and editing. Aiping Wang: writing — review and editing.
Corresponding authors
Ethics declarations
Ethics approval
All the animal experiments reported in this paper were approved by the Committee on the Ethics of Animal Experiments of the Yantai Institute of Materia Medica, and were performed in strict accordance with the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Consent to participate
Not applicable.
Consent for publication
All the authors provided consent for publication.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hui Liu, Yating Du, and Desheng Zhan contributed equally to this study.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, H., Du, Y., Zhan, D. et al. Oxaliplatin lipidated prodrug synergistically enhances the anti-colorectal cancer effect of IL12 mRNA. Drug Deliv. and Transl. Res. (2024). https://doi.org/10.1007/s13346-024-01540-x
Accepted:
Published:
DOI: https://doi.org/10.1007/s13346-024-01540-x