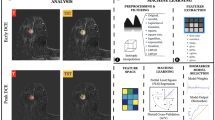
Abstract
Objectives
To explore the performance of multiparametric MRI-based radiomics in discriminating different human epidermal growth factor receptor 2 (HER2) expressing statuses (i.e., HER2-overexpressing, HER2-low-expressing, and HER2-zero-expressing) in breast cancer.
Methods
A total of 771 breast cancer patients from two institutions were retrospectively studied. Five-hundred-eighty-one patients from Institution I were divided into a training dataset (n1 = 407) and an independent validation dataset (n1 = 174); 190 patients from Institution II formed the external validation dataset. All patients were categorized into HER2-overexpressing, HER2-low-expressing, and HER2-zero-expressing groups based on pathologic examination. Multiparametric (including T2-weighted imaging with fat suppression [T2WI-FS], diffusion-weighted imaging [DWI], apparent diffusion coefficient [ADC], and dynamic contrast-enhanced [DCE]) MRI-based radiomics features were extracted and then selected from the training dataset using the least absolute shrinkage and selection operator (LASSO) regression. Three predictive models to discriminate HER2-overexpressing vs. others, HER2-low expressing vs. others, and HER2-zero-expressing vs. others were developed based on the selected features. The model performance was evaluated using the area under the receiver operating characteristic curve (AUC).
Results
Eleven radiomics features from DWI, ADC, and DCE; one radiomics feature from DWI; and 17 radiomics features from DWI, ADC, and DCE were selected to build three predictive models, respectively. In training, independent validation, and external validation datasets, radiomics models achieved AUCs of 0.809, 0.737, and 0.725 in differentiating HER2-overexpressing from others; 0.779, 0.778, and 0.782 in differentiating HER2-low-expressing from others; and 0.889, 0.867, and 0.813 in differentiating HER2-zero-expressing from others, respectively.
Conclusions
Multiparametric MRI-based radiomics model may preoperatively predict HER2 statuses in breast cancer patients.
Clinical relevance statement
The MRI-based radiomics models could be used to noninvasively identify the new three-classification of HER2 expressing status in breast cancer, which is helpful to the decision-making for HER2-target therapies.
Key Points
• Detecting HER2-overexpressing, HER2-low-expressing, and HER2-zero-expressing status in breast cancer patients is crucial for determining candidates for anti-HER2 therapy.
• Radiomics features from multiparametric MRI significantly differed among HER2-overexpressing, HER2-low expressing, and HER2-zero-expressing breast cancers.
• Multiparametric MRI-based radiomics could preoperatively evaluate three different HER2-expressing statuses and help to determine potential candidates for anti-HER2 therapy in breast cancer patients.




Similar content being viewed by others
Abbreviations
- ADC:
-
Apparent diffusion coefficient
- AUC:
-
Area under the curve
- DCE:
-
Dynamic contrast-enhanced
- DWI:
-
Diffusion-weighted imaging
- ER:
-
Estrogen receptor
- FS:
-
Fat suppression
- HER-2:
-
Human epidermal growth factor receptor-2
- ICC:
-
Intra-class correlation coefficient
- IHC:
-
Immunohistochemistry
- ISH:
-
In situ hybridization
- LASSO:
-
Least absolute shrinkage and selection operator
- PR:
-
Progesterone receptor
- ROC:
-
Receiver operating characteristic
- T1WI:
-
T1-weighted imaging
- T2WI:
-
T2-weighted imaging
- VOI:
-
Volume of interest
References
Siegel RL, Miller KD, Fuchs HE, Jemal A (2022) Cancer statistics, 2022. CA Cancer J Clin 72:7–33
Denkert C, Seither F, Schneeweiss A et al (2021) Clinical and molecular characteristics of HER2-low-positive breast cancer: pooled analysis of individual patient data from four prospective, neoadjuvant clinical trials. Lancet Oncol 22:1151–1161
Modi S, Jacot W, Yamashita T et al (2022) Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N Engl J Med 387:9–20
Tarantino P, Hamilton E, Tolaney SM et al (2020) HER2-low breast cancer: pathological and clinical landscape. J Clin Oncol 38:1951–1962
Miglietta F, Griguolo G, Bottosso M et al (2021) Evolution of HER2-low expression from primary to recurrent breast cancer. NPJ Breast Cancer 7:137
Fan P, Xu K (2023) Antibody-drug conjugates in breast cancer: marching from HER2-overexpression into HER2-low. Biochim Biophys Acta Rev Cancer 1878:188849
Miglietta F, Griguolo G, Bottosso M et al (2022) HER2-low-positive breast cancer: evolution from primary tumor to residual disease after neoadjuvant treatment. NPJ Breast Cancer 8:66
Prat A, Bardia A, Curigliano G et al (2022) An overview of clinical development of agents for metastatic or advanced breast cancer without ERBB2 amplification (HER2-low). JAMA Oncol https://doi.org/10.1001/jamaoncol.2022.4175
Loibl S, Gianni L (2017) HER2-positive breast cancer. Lancet 389:2415–2429
Bitencourt AGV, Gibbs P, Rossi Saccarelli C et al (2020) MRI-based machine learning radiomics can predict HER2 expression level and pathologic response after neoadjuvant therapy in HER2 overexpressing breast cancer. EBioMedicine 61:103042
Kazerouni AS, Hormuth DA 2nd, Davis T et al (2022) Quantifying tumor heterogeneity via MRI habitats to characterize microenvironmental alterations in HER2+ breast cancer. Cancers (Basel) 14:1837
Fang C, Zhang J, Li J et al (2022) Clinical-radiomics nomogram for identifying HER2 status in patients with breast cancer: a multicenter study. Front Oncol 12:922185
Xu A, Chu X, Zhang S et al (2022) Development and validation of a clinicoradiomic nomogram to assess the HER2 status of patients with invasive ductal carcinoma. BMC Cancer 22:872
Zhou J, Tan H, Li W et al (2021) Radiomics signatures based on multiparametric MRI for the preoperative prediction of the HER2 status of patients with breast cancer. Acad Radiol 28:1352–1360
Ramtohul T, Djerroudi L, Lissavalid E et al (2023) Multiparametric MRI and radiomics for the prediction of HER2-zero, -low, and -positive breast cancers. Radiology 308:e222646
Bian X, Du S, Yue Z et al (2023) Potential antihuman epidermal growth factor receptor 2 target therapy beneficiaries: the role of MRI-based radiomics in distinguishing human epidermal growth factor receptor 2-low status of breast cancer. J Magn Reson Imaging 58:1603–1614
Wolff AC, Hammond MEH, Allison KH et al (2018) Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. Arch Pathol Lab Med 142:1364–1382
Peng S, Chen L, Tao J et al (2021) Radiomics analysis of multi-phase DCE-MRI in predicting tumor response to neoadjuvant therapy in breast cancer. Diagnostics (Basel) 11:2086
Ellingson BM, Zaw T, Cloughesy TF et al (2012) Comparison between intensity normalization techniques for dynamic susceptibility contrast (DSC)-MRI estimates of cerebral blood volume (CBV) in human gliomas. J Magn Reson Imaging 35:1472–1477
van Griethuysen JJM, Fedorov A, Parmar C et al (2017) Computational radiomics system to decode the radiographic phenotype. Cancer Res 77:e104–e107
Tarantino P, Viale G, Press MF et al (2023) ESMO expert consensus statements (ECS) on the definition, diagnosis, and management of HER2-low breast cancer. Ann Oncol 34:645–659
Li H, Zhu Y, Burnside ES et al (2016) Quantitative MRI radiomics in the prediction of molecular classifications of breast cancer subtypes in the TCGA/TCIA data set. NPJ Breast Cancer 2:16012
Li C, Song L, Yin J (2021) Intratumoral and peritumoral radiomics based on functional parametric maps from breast DCE-MRI for prediction of HER-2 and Ki-67 status. J Magn Reson Imaging 54:703–714
Song L, Li C, Yin J (2021) Texture analysis using semiquantitative kinetic parameter maps from DCE-MRI: preoperative prediction of HER2 status in breast cancer. Front Oncol 11:675160
Liu Z, Li Z, Qu J et al (2019) Radiomics of multiparametric MRI for pretreatment prediction of pathologic complete response to neoadjuvant chemotherapy in breast cancer: a multicenter study. Clin Cancer Res 25:3538–3547
Takegawa N, Tsurutani J, Kawakami H et al (2019) [fam-] trastuzumab deruxtecan, antitumor activity is dependent on HER2 expression level rather than on HER2 amplification. Int J Cancer 145:3414–3424
Zhang H, Peng Y (2022) Current biological, pathological and clinical landscape of HER2-low breast cancer. Cancers (Basel) 15:126
Acknowledgements
The authors thank the colleagues from Shantou Central Hospital and Sun Yat-Sen Memorial Hospital for their constructive suggestions in the conception and completion of this work.
Funding
This study has received funding from the National Natural Science Foundation of China (82102130, 12126610), R&D project of Pazhou Lab (Huangpu) under Grant 2023K0606, Guangdong Basic and Applied Basic Research Foundation (2023A1515011305), Guangdong Medical Research Foundation (B2023426), Guangzhou Basic and Applied Basic Research Foundation (2023A04J2112), and Xinjiang Uygur Autonomous Region Tianshan Talent Youth Science and Technology Top Talent Project (2022TSYCJC0011).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Guarantor
The scientific guarantor of this publication is Xiang Zhang.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
One author (Zehong Yang) has statistical expertise.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained from the Institutional Review Board of Shantou Central Hospital (Shantou, China) ([2022] Research 072), Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University (Guangzhou, China) (SYSKY-2023–788-01).
Study subjects or cohorts overlap
No study subject or cohort has been previously reported in this study.
Methodology
• retrospective
• diagnostic study
• multicenter study
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zheng, S., Yang, Z., Du, G. et al. Discrimination between HER2-overexpressing, -low-expressing, and -zero-expressing statuses in breast cancer using multiparametric MRI-based radiomics. Eur Radiol (2024). https://doi.org/10.1007/s00330-024-10641-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00330-024-10641-7