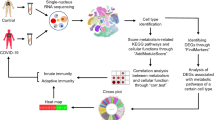
Abstract
Objective
This study aims to investigate the role of Acyl-CoA synthetase 4 (ACSL4) in mediating mitochondrial fatty acid metabolism and dendritic cell (DC) antigen presentation in the immune response associated with asthma.
Methods
RNA sequencing was employed to identify key genes associated with mitochondrial function and fatty acid metabolism in DCs. ELISA was employed to assess the levels of fatty acid metabolism in DCs. Mitochondrial morphology was evaluated using laser confocal microscopy, structured illumination microscopy, and transmission electron microscopy. Flow cytometry and immunofluorescence were utilized to detect changes in mitochondrial superoxide generation in DCs, followed by immunofluorescence co-localization analysis of ACSL4 and the mitochondrial marker protein COXIV. Subsequently, pathological changes and immune responses in mouse lung tissue were observed. ELISA was conducted to measure the levels of fatty acid metabolism in lung tissue DCs. qRT-PCR and western blotting were employed to respectively assess the expression levels of mitochondrial-associated genes (ATP5F1A, VDAC1, COXIV, TFAM, iNOS) and proteins (ATP5F1A, VDAC1, COXIV, TOMM20, iNOS) in lung tissue DCs. Flow cytometry was utilized to analyze changes in the expression of surface antigens presented by DCs in lung tissue, specifically the MHCII molecule and the co-stimulatory molecules CD80/86.
Results
The sequencing results reveal that ACSL4 is a crucial gene regulating mitochondrial function and fatty acid metabolism in DCs. Inhibiting ACSL4 reduces the levels of fatty acid oxidases in DCs, increases arachidonic acid levels, and decreases A-CoA synthesis. Simultaneously, ACSL4 inhibition leads to an increase in mitochondrial superoxide production (MitoSOX) in DCs, causing mitochondrial rupture, vacuolization, and sparse mitochondrial cristae. In mice, ACSL4 inhibition exacerbates pulmonary pathological changes and immune responses, reducing the fatty acid metabolism levels within lung tissue DCs and the expression of mitochondria-associated genes and proteins. This inhibition induces an increase in the expression of MHCII antigen presentation molecules and co-stimulatory molecules CD80/86 in DCs.
Conclusions
The research findings indicate that ACSL4-mediated mitochondrial fatty acid metabolism and dendritic cell antigen presentation play a crucial regulatory role in the immune response of asthma. This discovery holds promise for enhancing our understanding of the mechanisms underlying asthma pathogenesis and potentially identifying novel targets for its prevention and treatment.











Similar content being viewed by others
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AA:
-
Arachidonic acid
- ACSL4:
-
Acyl-CoA synthetase 4
- A-CoA:
-
Acyl-coenzyme A
- BALF:
-
Bronchoalveolar lavage fluid
- DC:
-
Dendritic cell
- DEGs:
-
Differentially expressed genes
- FAO:
-
Fatty acid oxidases
- GO:
-
Gene ontology
- IACUC:
-
Institutional animal care and use committee
- KEGG:
-
Kyoto encyclopedia of genes and genomes
- MAM:
-
Mitochondria-associated membrane
- MHCII:
-
Major histocompatibility complex class II
- MOC:
-
Mander's overlap coefficient
- PCC:
-
Pearson’s correlation coefficient
- PUFA:
-
Polyunsaturated fatty acids
- qRT-PCR:
-
Quantitative real-time PCR
- SEM:
-
Standard error of the mean
- SPF:
-
Specific pathogen-free
References
Shipp CL, Gergen PJ, Gern JE, Matsui EC, Guilbert TW. Asthma management in children. J Allergy Clin Immunol Pract. 2023;11(1):9–18. https://doi.org/10.1016/j.jaip.2022.10.031.
Pijnenburg MW, Nantanda R. Rising and falling prevalence of asthma symptoms. Lancet. 2021;398(10311):1542–3. https://doi.org/10.1016/S0140-6736(21)01823-7.
Asher MI, Rutter CE, Bissell K, et al. Worldwide trends in the burden of asthma symptoms in school-aged children: Global Asthma Network Phase I cross-sectional study. Lancet. 2021;398(10311):1569–80. https://doi.org/10.1016/S0140-6736(21)01450-1.
Lajiness JD, Cook-Mills JM. Catching our breath: updates on the role of dendritic cell subsets in asthma. Adv Biol (Weinh). 2023;7(6):e2200296. https://doi.org/10.1002/adbi.202200296.
Haczku A. The dendritic cell niche in chronic obstructive pulmonary disease. Respir Res. 2012;13(1):80. https://doi.org/10.1186/1465-9921-13-80.
Furlong-Silva J, Cook PC. Fungal-mediated lung allergic airway disease: the critical role of macrophages and dendritic cells. PLoS Pathog. 2022;18(7):e1010608. https://doi.org/10.1371/journal.ppat.1010608.
Upham JW, Xi Y. Dendritic cells in human lung disease: recent advances. Chest. 2017;151(3):668–73. https://doi.org/10.1016/j.chest.2016.09.030.
Bryant N, Muehling LM. T-cell responses in asthma exacerbations. Ann Allergy Asthma Immunol. 2022;129(6):709–18. https://doi.org/10.1016/j.anai.2022.07.027.
Kopf M, Schneider C, Nobs SP. The development and function of lung-resident macrophages and dendritic cells. Nat Immunol. 2015;16(1):36–44. https://doi.org/10.1038/ni.3052.
Humeniuk P, Dubiela P, Hoffmann-Sommergruber K. Dendritic cells and their role in allergy: uptake, proteolytic processing and presentation of allergens. Int J Mol Sci. 2017. https://doi.org/10.3390/ijms18071491.
Akdis CA, Arkwright PD, Brüggen MC, et al. Type 2 immunity in the skin and lungs. Allergy. 2020;75(7):1582–605. https://doi.org/10.1111/all.14318.
Munson PV, Adamik J, Hartmann FJ, et al. Polyunsaturated fatty acid-bound α-fetoprotein promotes immune suppression by altering human dendritic cell metabolism. Cancer Res. 2023;83(9):1543–57. https://doi.org/10.1158/0008-5472.CAN-22-3551.
Herber DL, Cao W, Nefedova Y, et al. Lipid accumulation and dendritic cell dysfunction in cancer. Nat Med. 2010;16(8):880–6. https://doi.org/10.1038/nm.2172.
Rehman A, Hemmert KC, Ochi A, et al. Role of fatty-acid synthesis in dendritic cell generation and function. J Immunol. 2013;190(9):4640–9. https://doi.org/10.4049/jimmunol.1202312.
Zhao F, Xiao C, Evans KS, et al. Paracrine Wnt5a-β-catenin signaling triggers a metabolic program that drives dendritic cell tolerization. Immunity. 2018;48(1):147-60.e7. https://doi.org/10.1016/j.immuni.2017.12.004.
Mehta MM, Weinberg SE, Chandel NS. Mitochondrial control of immunity: beyond ATP. Nat Rev Immunol. 2017;17(10):608–20. https://doi.org/10.1038/nri.2017.66.
Ellis JM, Frahm JL, Li LO, Coleman RA. Acyl-coenzyme A synthetases in metabolic control. Curr Opin Lipidol. 2010;21(3):212–7. https://doi.org/10.1097/mol.0b013e32833884bb.
Chen WC, Wang CY, Hung YH, Weng TY, Yen MC, Lai MD. Systematic analysis of gene expression alterations and clinical outcomes for long-chain acyl-coenzyme a synthetase family in cancer. PLoS ONE. 2016;11(5):e0155660. https://doi.org/10.1371/journal.pone.0155660.
Kuwata H, Nakatani E, Shimbara-Matsubayashi S, Ishikawa F, Shibanuma M, Sasaki Y, Yoda E, Nakatani Y, Hara S. Long-chain acyl-CoA synthetase 4 participates in the formation of highly unsaturated fatty acid-containing phospholipids in murine macrophages. Biochim Biophys Acta Mol Cell Biol Lipids. 2019;1864(11):1606–18. https://doi.org/10.1016/j.bbalip.2019.07.013.
Fujimoto Y, Itabe H, Kinoshita T, et al. Involvement of ACSL in local synthesis of neutral lipids in cytoplasmic lipid droplets in human hepatocyte HuH7. J Lipid Res. 2007;48(6):1280–92. https://doi.org/10.1194/jlr.M700050-JLR200.
Kuwata H, Hara S. Role of acyl-CoA synthetase ACSL4 in arachidonic acid metabolism. Prostaglandins Other Lipid Mediat. 2019;144:106363. https://doi.org/10.1016/j.prostaglandins.2019.106363.
Wang Y, Liao K, Liu B, et al. GITRL on dendritic cells aggravates house dust mite-induced airway inflammation and airway hyperresponsiveness by modulating CD4+ T cell differentiation. Respir Res. 2021;22(1):46. https://doi.org/10.1186/s12931-020-01583-x.
Yuan H, Li X, Zhang X, Kang R, Tang D. Identification of ACSL4 as a biomarker and contributor of ferroptosis. Biochem Biophys Res Commun. 2016;478(3):1338–43. https://doi.org/10.1016/j.bbrc.2016.08.124.
Jiang H, Zhang XW, Liao QL, Wu WT, Liu YL, Huang WH. Electrochemical monitoring of paclitaxel-induced ROS release from mitochondria inside single cells. Small. 2019;15(48):e1901787. https://doi.org/10.1002/smll.201901787.
Mogilenko DA, Haas JT, L’homme L, et al. Metabolic and innate immune cues merge into a specific inflammatory response via the UPR. Cell. 2019;178(1):263.
Qin T, Feng D, Zhou B, Bai L, Zhou S, Du J, Xu G, Yin Y. Melatonin attenuates lipopolysaccharide-induced immune dysfunction in dendritic cells. Int Immunopharmacol. 2023;120:110282. https://doi.org/10.1016/j.intimp.2023.110282.
Cools N, Ponsaerts P, Van Tendeloo VF, Berneman ZN. Balancing between immunity and tolerance: an interplay between dendritic cells, regulatory T cells, and effector T cells. J Leukoc Biol. 2007;82(6):1365–74. https://doi.org/10.1189/jlb.0307166.
van Helden MJ, Lambrecht BN. Dendritic cells in asthma. Curr Opin Immunol. 2013;25(6):745–54. https://doi.org/10.1016/j.coi.2013.10.002.
Liu S, Fan S, Wang Y, et al. ACSL4 serves as a novel prognostic biomarker correlated with immune infiltration in Cholangiocarcinoma. BMC Cancer. 2023;23(1):444. https://doi.org/10.1186/s12885-023-10903-5.
Grube J, Woitok MM, Mohs A, Erschfeld S, Lynen C, Trautwein C, Otto T. ACSL4-dependent ferroptosis does not represent a tumor-suppressive mechanism but ACSL4 rather promotes liver cancer progression. Cell Death Dis. 2022;13(8):704. https://doi.org/10.1038/s41419-022-05137-5.
Ibrahim J, Nguyen AH, Rehman A, et al. Dendritic cell populations with different concentrations of lipid regulate tolerance and immunity in mouse and human liver. Gastroenterology. 2012;143(4):1061–72. https://doi.org/10.1053/j.gastro.2012.06.003.
Killion EA, Reeves AR, El Azzouny MA, et al. A role for long-chain acyl-CoA synthetase-4 (ACSL4) in diet-induced phospholipid remodeling and obesity-associated adipocyte dysfunction. Mol Metab. 2018;9:43–56. https://doi.org/10.1016/j.molmet.2018.01.012.
Krawczyk CM, Holowka T, Sun J, et al. Toll-like receptor-induced changes in glycolytic metabolism regulate dendritic cell activation. Blood. 2010;115(23):4742–9. https://doi.org/10.1182/blood-2009-10-249540.
Wang L, Pino-Lagos K, de Vries VC, Guleria I, Sayegh MH, Noelle RJ. Programmed death 1 ligand signaling regulates the generation of adaptive Foxp3+CD4+ regulatory T cells. Proc Natl Acad Sci U S A. 2008;105(27):9331–6. https://doi.org/10.1073/pnas.0710441105.
Miao Z, Tian W, Ye Y, et al. Hsp90 induces Acsl4-dependent glioma ferroptosis via dephosphorylating Ser637 at Drp1. Cell Death Dis. 2022;13(6):548. https://doi.org/10.1038/s41419-022-04997-1.
Xu S, He Y, Lin L, Chen P, Chen M, Zhang S. The emerging role of ferroptosis in intestinal disease. Cell Death Dis. 2021;12(4):289. https://doi.org/10.1038/s41419-021-03559-1.
Kuwata H, Tomitsuka Y, Yoda E, Hara S. Role of ACSL4 in the chemical-induced cell death in human proximal tubule epithelial HK-2 cells. 2022. Biosci Rep. https://doi.org/10.1042/BSR20212433.
Wu L, Yan Z, Jiang Y, Chen Y, Du J, Guo L, Xu J, Luo Z, Liu Y. Metabolic regulation of dendritic cell activation and immune function during inflammation. Front Immunol. 2023;14:1140749. https://doi.org/10.3389/fimmu.2023.1140749.
Zeyda M, Säemann MD, Stuhlmeier KM, Mascher DG, Nowotny PN, Zlabinger GJ, Waldhäusl W, Stulnig TM. Polyunsaturated fatty acids block dendritic cell activation and function independently of NF-kappaB activation. J Biol Chem. 2005;280(14):14293–301. https://doi.org/10.1074/jbc.M410000200.
Carlsson JA, Wold AE, Sandberg AS, Östman SM. The Polyunsaturated fatty acids arachidonic acid and docosahexaenoic acid induce mouse dendritic cells maturation but reduce T-Cell responses in vitro. PLoS ONE. 2015;10(11):e0143741. https://doi.org/10.1371/journal.pone.0143741.
Dang D, Zhang C, Meng Z, Lv X, Li Z, Wei J, Wu H. Integrative analysis links ferroptosis to necrotizing enterocolitis and reveals the role of ACSL4 in immune disorders. iScience. 2022;25(11):105406. https://doi.org/10.1016/j.isci.2022.105406.
Acknowledgements
This study was supported by the Scientific Natural Science Foundation of Chongqing (cstc2020jcyj-msxmX0615), Youth Project of Scientific and Technological Research of Chongqing Municipal Education Commission (KJQN202200411), Chongqing Municipal Health Commission—Joint Scientific Research Project (2023QNXM015) and China Postdoctoral Science Foundation (2021MD703923) and the Graduate Student Research and Innovation Project of Chongqing Municipality (No.CYB22211).
Funding
Scientific Natural Science Foundation of Chongqing, cstc2020jcyj-msxmX0615, Youth Project of Scientific and Technological Research of Chongqing Municipal Education Commission, KJQN202200411, Chongqing Municipal Health Commission—Joint Scientific Research Project, 2023QNXM015, China Postdoctoral Science Foundation, 2021MD703923, Graduate Student Research and Innovation Project of Chongqing Municipality, CYB222111.
Author information
Authors and Affiliations
Contributions
YL, EML, ZXL, ZF, BL and FXD were responsible for the overall conception and design of the study. YL performed most of the experiments and the data analysis, collected the data, and wrote the manuscript. YL, WLF, JYY, BL and FXD revising the manuscript. JYX, YYR, YHL and MZ contributed to the sample collection. All authors reviewed, edited and approved the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethics approval and consent to participate
The study received approval from the Ethics Committee of the Children's Hospital of Chongqing Medical University (Approval No. 2022-004).
Consent for publication
Not applicable.
Additional information
Responsible Editor: L. Li.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, Y., Fu, W., Xiang, J. et al. Long-chain acyl-CoA synthetase 4-mediated mitochondrial fatty acid metabolism and dendritic cell antigen presentation. Inflamm. Res. 73, 819–839 (2024). https://doi.org/10.1007/s00011-024-01868-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-024-01868-7